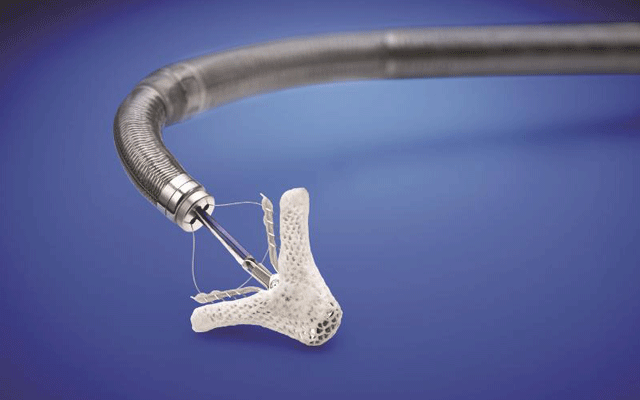
Abbott has announced that the US Centers for Medicare & Medicaid Services (CMS) has revised its National Coverage Determination (NCD) to expand coverage for transcatheter edge-to-edge repair (TEER), also referred to as transcatheter mitral valve repair (TMVR), to include patients with secondary (or functional) mitral regurgitation (MR) resulting from heart failure.
The decision increases the number of people eligible for insurance coverage for mitral valve repair with MitraClip, enabling broader access to the device. As the first and only TEER device approved by the US Food and Drug Administration (FDA) and reimbursed by Medicare for primary mitral regurgitation, physicians have increasingly relied on the therapy to improve survival and quality of life for their patients. Today’s decision improves insurance coverage for people with secondary MR who need treatment with MitraClip.
“Secondary mitral regurgitation generally impacts older individuals suffering from heart failure who rely on Medicare for their healthcare coverage,” said Neil Moat, chief medical officer of Abbott’s structural heart business. “CMS’ decision to expand coverage for MitraClip marks a pivotal moment for people seeking a minimally invasive option that reduces mitral regurgitation and significantly improves their quality of life and chances of survival.”
Following MitraClip’s original approval by the FDA in late 2013, CMS provided coverage for Medicare patients with primary (degenerative) mitral regurgitation who needed treatment with MitraClip. Similarly, this revised NCD comes after the FDA’s 2019 expanded indication for MitraClip to treat people with secondary MR. The decision also follows the highest MR reduction data for MitraClip reported to date: Recent 2020 data demonstrated MR reduction was consistently achieved with MitraClip in patients with either primary MR (to ≤1+ in 87.1%) or secondary MR (to ≤1+ in 90.1%) at 30 days.
“Approximately four million Americans suffer from MR, and it’s estimated that two to three times as many patients may benefit from MitraClip for secondary mitral regurgitation than those for the primary form of the disease,” said Michael Dale, senior vice president of Abbott’s structural heart business. “We’ve worked tirelessly, for nearly two decades, to make MitraClip’s leading technology available to patients suffering from mitral regurgitation, and CMS’ expanded coverage allows our safe, effective and potentially life-saving treatment option to be available to the many more people who could benefit from our minimally invasive therapy.”












