Tag: Abbott
Navitor TAVI valve picks up expanded CE mark for low-risk patients
Abbott’s Navitor transcatheter aortic valve implantation (TAVI) system has received CE mark for the ...
Abbott to begin TECTONIC trial of IVL therapy prior to coronary stenting
Abbott has announced that the US Food and Drug Administration (FDA) has approved an investigatio...
First patient procedures performed with Abbott’s balloon-expandable TAVI system
Abbott has announced the first patient procedures with its investigational transcatheter aortic ...
Two-year LIFE-BTK data show sustained benefits of drug-eluting resorbable scaffold for below-the-knee arteries
Presented today, late-breaking data from the second year of the LIFE-BTK clinical trial demonstr...
TCT 2024: Drug-eluting resorbable scaffold proves cost effective at one year in LIFE-BTK analysis
A retrospective economic analysis of the LIFE-BTK trial has demonstrated the one-year cost-effec...
Abbott’s Navitor Vision TAVI valve rolled out in UK and Ireland
Abbott is rolling out the latest iteration of its Navitor transcatheter aortic valve implantatio...
Abbott’s Esprit BTK scaffold system given US FDA approval for CLTI treatment
Abbott has announced that the US Food and Drug Administration (FDA) has approved the Esprit BTK ever...
TriClip TEER system for TR repair given US FDA approval
Abbott has announced that the US Food and Drug Administration (FDA) has approved the company's TriCl...
TriClip gets positive vote from US FDA advisory panel
The Circulatory System Devices Panel of the Medical Devices Advisory Committee for the US Food and D...
TCT 2023: LIFE-BTK breathes life into drug-eluting resorbable scaffolds in breakthrough for below-the-knee arteries
Results of the LIFE-BTK randomised controlled trial have just been presented at TCT 2023 (23–26 ...
Lars Søndergaard joins Abbott’s structural heart business
Lars Søndergaard has left his role as professor of cardiology at Rigshospitalet, Copenhagen Universi...
Remote monitoring improves survival in heart failure patients analysis finds
Abbott has today announced new data that found monitoring patients remotely with haemo...
One-year real-world data “promising” for transcatheter tricuspid repair
One-year insights from the bRIGHT registry, detailing real-world results of tricuspid transcath...
US FDA approves use of Navitor TAVI valve in high-risk aortic stenosis patients
The US Food and Drug Administration (FDA) has approved the Navitor (Abbott) transcatheter aortic val...
Abbott’s CardioMEMS HF system receives FDA approval for use in earlier-stage heart failure
Abbott has announced that the US Food and Drug Administration (FDA) has approved an expanded indicat...
Abbott’s Nick West points to future role of AI in the cath lab
Abbott has released a second round of insights from its Beyond Intervention global research prog...
CUTTING-EDGE registry data sheds light on surgical outcomes following failed transcatheter edge-to-edge repair
Gilbert Tang (New York, USA) talks to Cardiovascular News about the key findings from the CUTTIN...
EXPAND registry outcomes of new MitraClip devices in line with COAPT
Jörg Hausleiter (Munich, Germany) talks to Cardiovascular News about the one-year findings of ...
Transcatheter tricuspid valve repair shows promise for reducing heart failure hospitalisations
In JACC: Heart Failure, Mathias Orban (Medizinische Klinik und Poliklinik I, Klinikum der Univer...
CE mark for transcatheter system that uses same clip technology as MitraClip
Abbott has received the CE mark for its TriClip transcatheter tricuspid valve repair system. It ...
Virtual ACC: Newer generations of MitraClip associated with greater reduction in mitral regurgitation than previous version
Today at the American College of Cardiology/World Congress of Cardiology’s virtual scientific se...
FDA Breakthrough Device Designation for fully implantable left ventricular assist system
The US Food and Drug Administration (FDA) has granted Breakthrough Device Designation to Abbott ...
Abbott becomes first company in world to have transcatheter mitral implantation device on market
The Tendyne transcatheter mitral valve implantation (TMVI) system (Abbott) has received the CE mark,...
Abbott starts trial to evaluate TriClip for percutaneous repair of tricuspid regurgitation
Abbott has announced its TRILUMINATE pivotal trial to evaluate the safety and effectiveness of t...
ESC 2019: Two-year findings of MITRA-FR continue to show no prognosis benefit with MitraClip
At the European Society of Cardiology Congress (ESC 2019; 31 August–4 September, Paris, France),...
Fourth-generation MitraClip receives FDA approval
According to a statement, Abbott has announced it has received US Food and Drug Administration (...
MitraClip can be used to treat secondary mitral regurgitation in the USA
The US FDA has approved a new indication for percutaneous edge-to-edge repair (MitraClip, Abbott...
CRT 2019: US FDA approves resting full-cycle ratio for measuring severity of coronary artery blockages
Abbott has announced the US FDA clearance of the resting full-cycle ratio (RFR) diagnostic test to i...
Abbott to acquire Cephea Valve Technologies
It was recently announced that Abbott has exercised its option to purchase Cephea Valve Technologies...
FDA approves world’s first device for treatment of premature babies and newborns with an opening in their hearts
The US Food and Drug Administration (FDA) has approved the Amplatzer Piccolo Occluder (Abbott), ...
US FDA approve HeartMate 3 heart pump for advanced heart failure patients not eligible for a heart transplant
The HeartMate 3 left ventricular assist device has received US FDA approval as a destination the...
TCT 2018: One-year real-world results show Portico TAVI system reduces severe aortic stenosis
According to one-year results from the PORTICO I study, which were presented during a late-breaking ...
ESC 2018: MitraClip makes “absolutely no difference” for the prognosis of secondary mitral valve regurgitation
The first randomised controlled trial to compare percutaneous edge-to-edge repair (MitraClip, Ab...
Abbott launches trial of its Tendyne transcatheter mitral valve implantation device
Abbott has initiated a pivotal clinical study in the USA of its Tendyne transcatheter mitral val...
Next-generation of MitraClip approved for use in the USA
Abbott has received approval from the US FDA for a next-generation version of its MitraClip hear...
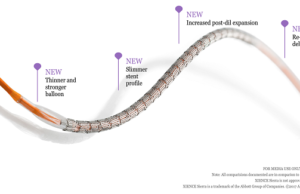
Xience Sierra approved for use in USA
Abbott has announced it has received approval from the US FDA for its Xience Sierra, the newest ...
FDA approves the world’s smallest mechanical heart valve
The US FDA has approved the Masters HP 15mm rotatable mechanical heart valve (Abbott), which a p...
First patient enrolled in trial reviewing 28-day DAPT with Xience
The first patient has been enrolled in a clinical trial evaluating 28 days of dual antiplatelet ...
TCT 2017: Advances in device technology needed to improve outcomes with Absorb
Data from the ABSORB IV study indicate that device thrombosis with the bioresorbable vascular sc...
MitraClip becomes first approved device for transcatheter mitral valve repair in Japan
Abbott has announced that Japan's Ministry of Health, Labour and Welfare (MHLW) has approved its...
Abbott’s Full MagLev HeartMate 3 LVAD receives FDA approval
The FDA has approved the Full MagLev HeartMate 3 (Abbott) left ventricular assist device for use...
US trial to evaluate safety and efficacy of Amplatzer for patent ductus arteriosus
Abbott has initiated a US pivotal clinical study evaluating the safety and effectiveness of a mo...
Millipede 50mm annuloplasty ring used in first cases
Millipede has announced the successful implantation of its newest 50mm Iris annuloplasty ring, w...
ACC 2017: CardioMEMS HF system effective in reducing heart failure hospitalisations and cost of care
Late-breaking clinical research presented at the American College of Cardiology 66th Annual Scie...
Terumo signs to acquire St Jude Medical and Abbott’s vascular closure business
Terumo has reached an agreement with Abbott and St Jude Medical to acquire certain products owned by...
St. Jude Medical and Abbott to sell portion of vascular closure and electrophysiology businesses to Terumo
Abbott and St. Jude Medical have announced an agreement in principle to sell certain products to...