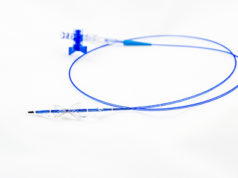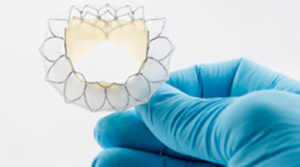
Neovasc has received both regulatory and ethics committee approval to initiate the Tiara transcatheter mitral valve replacement study (TIARA II) in Italy. The study will be a 115-patient, non-randomised, prospective clinical study that will evaluate the safety and performance of the Tiara transcatheter mitral valve with the Tiara transapical delivery system.
A press release notes that with these approvals now in place, it is expected that the first Italian TIARA II clinical study site will be initiated before the year end, with first enrolment anticipated early in the New Year. Approvals in additional geographies are expected in the first quarter of 2017. According to the press release, it is expected that data from this study will be used to file for CE mark approval for Tiara.
Alexei Marko, Neovasc chief executive officer, comments: “We have been very encouraged by the results to date with the Tiara device. Tiara has been successfully implanted in a wide range of patients including those with prosthetic aortic valves in place (both tissue and mechanical valves) and those with prior mitral valve surgical repairs, including mitral rings. With this approval, we look forward to beginning our CE mark study which offers a less invasive treatment option for patients determined to be high risk for surgery, suffering from severe mitral regurgitation, and to eventually have Tiara available as a treatment option for clinical use to treat this devastating disease.”
Tiara is a self-expanding mitral bioprosthesis specifically designed to treat mitral valve regurgitation by replacing the diseased valve. Conventional surgical treatments are only appropriate for about half of mitral regurgitation patients, who number an estimated four million in the USA with a similar number of patients affected throughout Europe. Tiara is implanted in the heart using a minimally invasive, transapical transcatheter approach and is designed to replace the diseased native mitral valve without the need for open-heart surgery or use of a cardiac bypass machine.