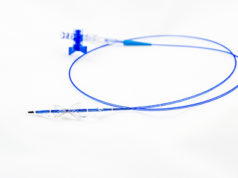
Neovasc has announced that the Institut für das Entgeltsystem im Krankenhaus (InEk)— the German Institute for the Hospital Remuneration System—has awarded its Neovasc Reducer, a CE-marked medical device for the treatment of refractory angina, NUB status 1 designation again for 2019. New examination and treatment methods (NUBs) comprise of novel and innovative medicines, medical products and procedures that can be applied by the hospital before they can be settled via flat rates or additional charges (KHEntgG).
A press release reports that the NUB process opens the path for negotiations between hospitals and health insurances on the reimbursement of new medical treatments in the German system. InEK, is responsible for prioritising new therapies in Germany through the NUB process.
InEK renewed the status of the Neovasc Reducer as status 1—the highest priority designation available. A NUB decision is valid for one year and can be renewed annually. This year, 159 German hospitals applied for the Reducer NUB status (compared to 107 German hospitals last year) and can now negotiate reimbursement coverage for the Neovasc Reducer therapy under the German health insurance system.
Fred Colen, CEO of Neovasc, comments: “We are excited to be able to continue treating German patients suffering from refractory angina with the Reducer and an almost 50% increase in the number of hospitals applying for NUB, which we believe is a strong metric to signal the growing adoption of the therapy in Germany. This positive development will allow us to continue our sales and therapy development efforts.”