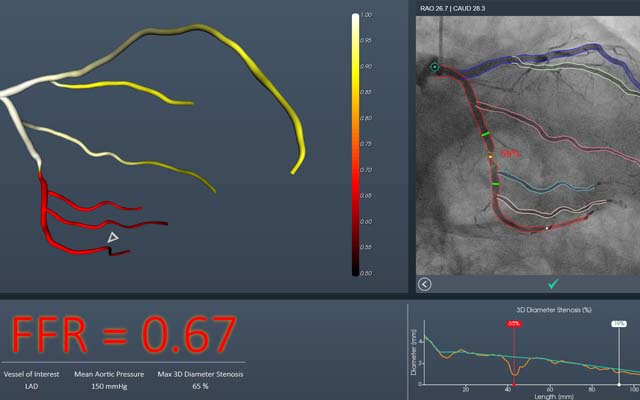
CathWorks has announced that its FAST-FFR trial is fully enrolled ahead of schedule. The trial is designed to evaluate efficacy of the CathWorks FFRangio system in terms of sensitivity and specificity when compared to conventional invasive fractional flow Reserve (FFR). Enrolment in the trial began on 27 September 2017 and enrolment of all 382 patients was completed just eight months later on 7 June 2018.
A press release reports that the FFRangio system is non-invasive and performed intra-procedurally, during coronary angiography. It is designed to enable physicians to objectively and cost-effectively determine if percutaneous coronary intervention (PCI) is indicated, without an additional intervention during the course of a routine diagnostic angiography. The FAST-FFR trial is being conducted at 10 cardiovascular centres around the world using various coronary angiography platforms. These sites include:
- OLV Aalst, Belgium
- Rabin Medical Center, Israel
- Rigs Hospital CPH, Denmark
- St Francis Hospital, USA
- University of Erlangen, Germany
- Stanford University, USA
- Share Zedek Medical Center
- Columbia University, USA
- HaSharon Medical Center, Israel
- University of Pennsylvania, USA
William F Fearon (Stanford University Medical Center, Stanford, USA) is the FAST-FFR trial principal investigator. He says: “As interventional cardiologists, we want objective multivessel physiologic measurements to make PCI decisions, which can be performed easily and with as low risk and cost as possible,” Dr. Fearon said. “We look forward to the data analysis and understanding how CathWorks FFRangio System data compares to pressure wire-derived FFR.”
Jim Corbett, CathWorks CEO, comments: “Early enrolment of the FAST-FFR trial population confirms interventional cardiologists’ excitement about cost-effectively adding a new level of objectivity in PCI decision-making.”
Following the completion of trial enrolment, CathWorks announced that it has submitted its FFRangio system to the US FDA for review and 510(k) market clearance. Corbett says; “The FAME trials clearly demonstrated the clinical and economic value of FFR in PCI decision-making. We are confident that the CathWorks FFRangio system meets a real need for patients and physicians, and we expect the system to make non-invasive, wire-free FFR possible in coronary angiograms; reducing wire-related risks and costs.”