Tag: FFRangio
FFRangio system receives US FDA clearance
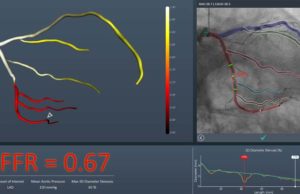
US FDA 510(k) clearance has been granted for the FFRangio System (CathWorks). The FFRangio syste...
TCT 2018: FFRangio may provide a faster and easier approach to physiological assessment
The FAST-FFR trial, which was presented at the 2018 Transcatheter Cardiovascular Therapeutics (T...
Enrolment in FAST-FFR trial completed early
CathWorks has announced that its FAST-FFR trial is fully enrolled ahead of schedule. The trial i...
CPT code for CathWorks’ 3D FFRangio wire free system
CathWorks has received a new CPT code 0523T for its non-invasive, 3D FFRangio system, which is d...