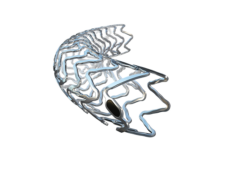
A press release from Biotronik highlights data from the BIOSOLVE-IV registry outlining procedural success and a positive safety profile at one year for its Magmaris Resorbable Magnesium Scaffold (RMS) device.
The target lesion failure (TLF) rate at 12 months was 4.7%. The press release also points out that Magmaris showed a very good safety profile, with cardiac death occurring in 0.1% of the patients and scaffold thrombosis in 0.6%. The device success rate was 97.1% and procedure success 98.8%.
Stefan Verheye (Antwerp Cardiovascular Institute, Antwerp, Belgium) presented results on the first 800 patients from the BIOSOLVE-IV registry cohort at EuroPCR 2019 (20–24 May, Paris, France). In the company’s press release he comments: “Overall, BIOSOLVE-IV confirmed low TLF rates from previous trials in a real world setting, including 17.8% NSTEMI patients and 5.6% patients with bifurcation lesions. When looking closer at the five scaffold thrombosis cases, we noticed that in four of them antiplatelet or anticoagulant therapy was interrupted early after scaffold implantation.”
The Magmaris Resorbable Magnesium Scaffold is not currently commercially available in the USA.