The American Medical Association (AMA) has issued a set of new Category III Current Procedural Terminology (CPT) codes for HeartFlow FFRct, a first-of-its-kind noninvasive technology designed to help clinicians diagnose and treat patients with suspected coronary artery disease (CAD).
The CPT code application was submitted collectively by the American College of Cardiology (ACC), the Society of Cardiovascular Computed Tomography (SCCT) and the Society for Cardiovascular Angiography and Interventions (SCAI).
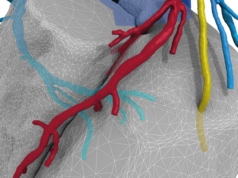
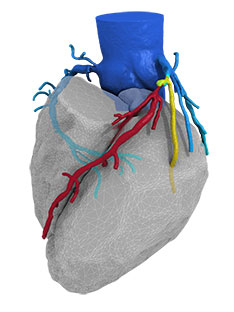
HeartFlow FFRct is intended to provide insight into both the extent of CAD and the impact of the disease on blood flow to the heart. HeartFlow FFRct is designed to enable clinicians to select a definitive, personalised treatment plan for each patient and reduce the need for additional invasive testing.
With the new CPT codes, hospitals and clinics utilising HeartFlow FFRct will be able to generate claims with both US Medicare and commercial payers when FFRct is ordered for patients with suspected CAD.
The new HeartFlow FFRct CPT codes will become effective on January 1, 2018. They include:
- 0501T – Noninvasive estimated coronary fractional flow reserve (FFR) derived from coronary computed tomography angiography data using computation fluid dynamics physiologic simulation software analysis of functional data to assess the severity of coronary artery disease; data preparation and transmission, analysis of fluid dynamics and simulated maximal coronary hyperemia, generation of estimated FFR model, with anatomical data review in comparison with estimated FFR model to reconcile discordant data, interpretation and report
- 0502T – Data preparation and transmission
- 0503T – Analysis of fluid dynamics and simulated maximal coronary hyperemia, and generation of estimated FFR model
- 0504T – Anatomical data review in comparison with estimated FFR model to reconcile discordant data, interpretation and report