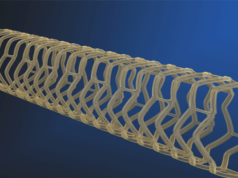
Reva has been granted CE mark approval for its Fantom drug-eluting bioresorbable coronary scaffold. Fantom is Reva’s first commercial product.
With this approval, Reva plans to commence selling in selected centres in Europe this financial quarter.
Data from patients enrolled in the Company’s FANTOM II clinical trial were used to support the CE mark application. The trial enrolled a total of 240 patients between March 2015 and March 2016. The major adverse cardiac event rate through six months for all 240 patients is 2.1%, which the company states “compares favourably to commercial first-generation bioresorbable scaffolds” in a press release. Reva and plans additional data releases at industry conferences in May and October of this year.