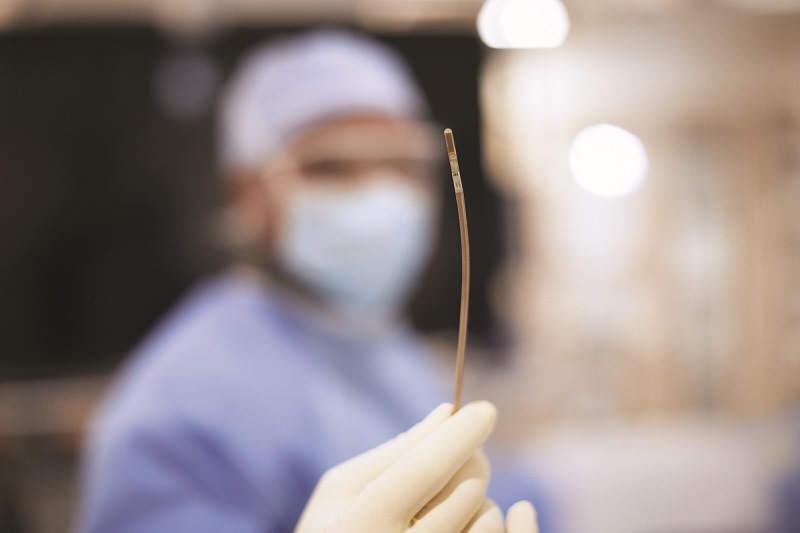
 Royal Philips has announced that Baptist Health’s Miami Cardiac & Vascular Institute (Miami, USA) has become the latest centre to begin treating patients with Verisight Pro, the company’s real-time 3D intracardiac echocardiography (ICE) catheter, during minimally-invasive image-guided procedures for structural heart disease.
Royal Philips has announced that Baptist Health’s Miami Cardiac & Vascular Institute (Miami, USA) has become the latest centre to begin treating patients with Verisight Pro, the company’s real-time 3D intracardiac echocardiography (ICE) catheter, during minimally-invasive image-guided procedures for structural heart disease.
According to a press release from Philips, using the VeriSight Pro ICE catheter, additional groups of patients can now be treated, such as those who are not suitable for transesophageal echocardiography (TEE), which typically requires heavy sedation or general anaesthesia.
Ramon Quesada and Bernardo Lopez-Sanabria are the first two interventional cardiologists to use this technology for Baptist Health.
“VeriSight Pro’s technology is a game changer, allowing us to give people who need this procedure a chance for a smoother and safer recovery,” said Quesada. “As physicians, we are always exploring new ways to care for our patients and provide them with the best treatment options for their long-term future.”
“This device allows our physicians to capture the very best possible live images and enable our team to deliver quality outcomes for our cardiac patients, so they can get back to their daily routines,” said Lopez-Sanabria. “I am honoured to be the first physician in South Florida to use this new technology and bring it to our community.”
Ultrasound imaging to guide structural heart repair and electrophysiology procedures has conventionally been via transthoracic echocardiography (TTE), in which an ultrasound probe is placed on the patient’s chest, or via TEE, which involves the insertion of an ultrasound probe into the patient’s oesophagus so that it lies close to their heart. According to Philips, TTE typically results in sub-optimal imaging, while TEE requires heavy sedation or general anaesthesia so that the patient can tolerate the procedure. To overcome these limitations, Philips ICE catheter utilises an ultra-miniature ultrasound probe mounted on the end of a 3mm diameter (9Fr) catheter, allowing it to be routed via the patient’s vasculature to inside their heart.
Philips’ FDA 510(k)-cleared 3D ICE catheter, VeriSight Pro, has the potential to improve the standard of care for structural heart disease and electrophysiology procedures and broaden the range of treatment options open to patients.