 Early experience with the Evoque (Edwards Lifesciences) tricuspid valve replacement system demonstrated a significant reduction in tricuspid regurgitation (TR) and symptoms in patients with clinically significant TR. This is according to 30-day results of the TRISCEND study, presented during a late-breaking clinical trial session at EuroPCR 2021 (18–20 May, virtual) by Susheel Kodali (Columbia University Medical Center, New York, USA).
Early experience with the Evoque (Edwards Lifesciences) tricuspid valve replacement system demonstrated a significant reduction in tricuspid regurgitation (TR) and symptoms in patients with clinically significant TR. This is according to 30-day results of the TRISCEND study, presented during a late-breaking clinical trial session at EuroPCR 2021 (18–20 May, virtual) by Susheel Kodali (Columbia University Medical Center, New York, USA).
Evoque is a tricuspid valve replacement system, designed to be deployed via a transfemoral approach. The design of the valve features atraumatic anchors which are used to engage the native tricuspid valve leaflets, under echocardiographic guidance. TRISCEND is a prospective, single-arm, multicentre study, seeking to evaluate the safety and performance of the device, in patients with symptomatic moderate TR. Endpoints studied include device and procedural success, TR reduction, and a composite of major adverse events (MAE) at 30 days.
A total of 56 patients were enrolled in the study, with a mean age of 79±8 years, 77% of whom were female. Of these patients, Kodali detailed, 90% had severe or greater TR. Follow-up data after 30 days were available for 47% of patients.
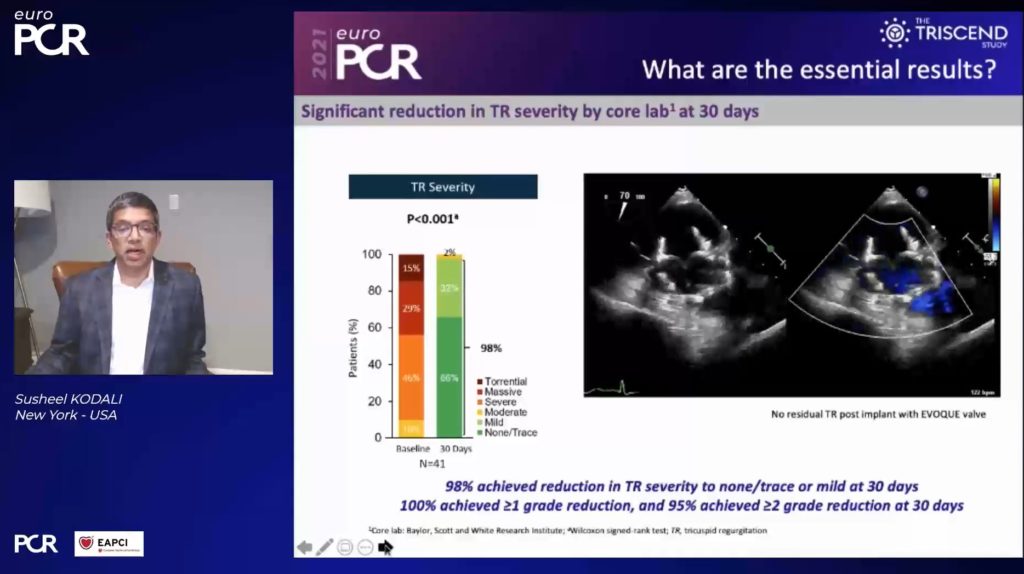
Device success, which was defined as device deployment and delivery system retrieval as intended, was achieved in 98% of patients, Kodali said, while procedural success, which was defined as device success without significant paravalvular leak (PVL), was achieved in 94% of patients. Echo corelab analysis revealed that 90% had severe or greater TR at baseline, with 44% classed as ‘massive or torrential’. At 30-day follow-up, 98% had mild or non-traced TR, with all patients achieving at least a one-grade reduction in TR, and 95% improving by at least two grades.
Two mortalities occurred within the 30-day follow-up, Kodali noted, while bleeding events occurred in 22.6% of patients. In total, 77.4% had no major adverse events at 30 days.
“All patients achieved significant improvements in clinical, functional and quality of life outcomes at 30 days,” Kodali said, adding that baseline NYHA class improved “significantly” among the cohort, with 84% having been NYHA class 3 or 4 at baseline, and 77% class 1 or 2 at the 30-day follow-up.
Concluding his presentation, Kodali commented that the Evoque tricuspid valve replacement system may be an important therapy for patients with severe tricuspid regurgitation, “which are often undertreated”.
“This study showed favourable 30-day results, with high device and procedural success rates—98% and 94% respectively—a significant reduction in TR severity, with 98% mild or less TR at 30-day follow-up,” Kodali commented.
He concluded: “The early experience with the Evoque transfemoral system demonstrated technical feasibility with an acceptable safety profile along with significant TR reduction and symptomatic improvement at 30 days in patients with clinically significant TR.”
A pivotal trial evaluating the use of the device has been initiated as a result of the study’s results, Kodali said.