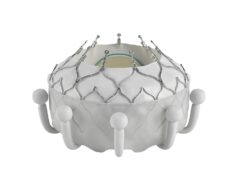
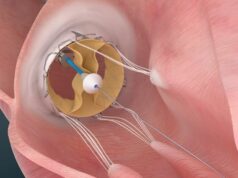
One-year results from the TRISCEND study, investigating the Evoque (Edwards Lifesciences) tricuspid valve replacement system, have demonstrated favourable safety, efficacy, echocardiographic and quality of life outcomes in patients with symptomatic tricuspid regurgitation (TR), investigators have reported.
Stephan Windecker (Bern University Hospital, Bern, Switzerland) presented the trial’s clinical, functional and echocardiographic outcomes at 12 months in a late-breaking trial session at PCR London Valves (27–29 November, London, UK), following on from encouraging results at both 30 days and six months.
TRISCEND is a prospective, multicentre, single-arm study, that has enrolled 176 patients with symptomatic, moderate or greater primary or secondary tricuspid regurgitation despite medical therapy at 20 sites across the USA, Canada, France and Switzerland.
In his presentation, Windecker detailed that the Evoque transcatheter valve replacement system consists of a self-expanding nitinol frame, with a pericardial bovine valve and fabric skirt, delivered through a 28Fr transfemoral delivery system, inserted transvenously.
Summarising the patient characteristics, Windecker described the trial’s population as elderly, with a mean age of 78.7, largely female (71%), and noted that these patients had frequent comorbidities and three quarters of the patients were highly symptomatic with New York Heart Association class III‒IV. Additionally, 92% of patients had atrial fibrillation (AF), 22% ascites, and 32% of patients had had prior pacemaker or implantable cardioverter defibrillator (ICD).
The Evoque implant procedure was performed successfully in 94.4% of patients, with a relatively “short” implant time of 71.6 minutes needed on average per patient. Discharge to home was achieved in 91.1% of patients, with the length of hospital stay averaging three days.
Turning to the clinical results, Windecker detailed that cardiovascular mortality, which stood at 1.7% at 30 days, totalled 9.4% at one year. Further to this, no incidence of myocardial infarction (MI) was reported, there was a low rate of stroke (0.6% at 30 days and 1.3% at one year). Non-elective tricuspid valve reinterventions were observed in 4% of patients at one year and severe bleeding was 16.9% at one month and 25.5% at one year.
There were no major cardiac structural complications and the composite major adverse event rate was 18.6% at 30 days and 30.2% at one year, Windecker reported. Pacemakers were implanted in 13.3% of patients within 30 days, but there was no further implant beyond 30 days. Furthermore, the results showed a 90.1% rate of survival at one year, alongside an 88.4% rate of freedom from heart failure hospitalisation.
Windecker also then presented serial core lab assessment of TR at both 30 days and one year versus baseline. He commented: “What you note is that the vast majority of patients that had severe, massive or torrential MR, in nearly 90% of cases at baseline was turned into an outcome where there was no, or only mild tricuspid regurgitation in 98% of patients. Of note, these changes were durable at all times of follow-up: 30 days, six months and one year, and there was the absence of any severe or more tricuspid regurgitation.
Outlining the echocardiographic results, which were adjudicated by core lab at one year, Windecker commented that there was evidence of right ventricular (RV) remodelling after Evoque implant, demonstrated by a change in mean RV end-diastolic mid diameter from 41.4mm at baseline to 35mm at one year and inferior vena cava (IVC) diameter was reduced from 27.6mm at baseline to 20.4mm at one year.
On functional and quality of life changes, Windecker commented that prior to the intervention, most of the patients were highly symptomatic, but after the intervention, 93% were oligosymptomatic with New York Heart Association (NYHA) classification I or II. “You see a remarkable difference in Kansas City Cardiomyopathy Questionnaire (KCCQ) score where a mean score of 46 increased to 17.7 with a change of 26,” noted Windecker. “To give you an impression, any change of more than 20 is considered large or a very large improvement in these patients, and there was also a highly significant difference in the improvements of six-minute walk test of 56 metres.”
Summarising the overall message from the presentation, Windecker commented that the findings at one year demonstrate “favourable survival and freedom from heart failure hospitalisation”.
“The core lab-adjudicated rates of TR reduction were remarkable, where 98% of patients had either no or only mild tricuspid regurgitation,” he added. “There was echocardiographic evidence of right ventricular remodelling and there were also significant clinically meaningful improvements in heart failure symptoms, and very beneficial development as it relates to quality of life measures, KCCQ score and the six-minute walk test.”
The results will be further investigated in the randomised TRISCEND II study, which will pit the Evoque device against medical therapy.