Tag: Terumo
Terumo announces US FDA clearance for OpusWave imaging system
Terumo Interventional Systems has received 510(k) clearance from the U.S. Food and Drug Administrati...
Terumo brings Medis QFR 3.0 to US market
Terumo Health Outcomes (THO), a division of Terumo Interventional Systems (TIS), has announced that ...
PCR e-Course 2020: Data for Ultimaster reinforce safety and efficacy of device
According to a press release, one-year total population clinical outcome data from e-ULTIMASTER—...
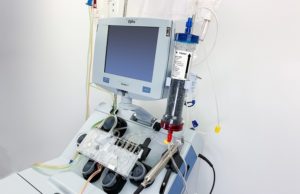
COVID-19: FDA authorise blood purification device for treating virus
The US Food and Drug Administration (FDA) issued an emergency use authorisation for a blood puri...
New randomised study investigating distal radial access, DISCO RADIAL, launches
Terumo has announced the launch of a new randomised clinical trial: the DIStal vs. COnventio...
Enrolment completed for MASTER DAPT trial
A significant milestone has been reached for a landmark study into the use of short du...
Virtue sirolimus-eluting balloon set for commercial release as Terumo and Orchestra BioMed enter global strategic partnership
Orchestra BioMed and Terumo have formed a global strategic partnership for the development and comme...
Terumo receives CE mark for Ultimaster Tansei drug-eluting stent
Terumo has received the CE mark for its Ultimaster Tansei drug-eluting stent. Building on the Ul...
First patient enrolled in new study of abbreviated DAPT post-stent in high bleeding risk patients
Gillian Jessurun (Treant Zorggroep, Scheper Hospital Emmen, The Netherlands) has enrolled the first ...
Terumo completes acquisition of vascular closure business and other assets of Abbott and St Jude Medical
Terumo has announced that it has completed its acquisition of certain assets owned by Abbott and...
Terumo signs to acquire St Jude Medical and Abbott’s vascular closure business
Terumo has reached an agreement with Abbott and St Jude Medical to acquire certain products owned by...
St. Jude Medical and Abbott to sell portion of vascular closure and electrophysiology businesses to Terumo
Abbott and St. Jude Medical have announced an agreement in principle to sell certain products to...
Terumo’s FineDuo microcatheter receives CE mark
FineDuo—a low profile, multifunctional, dual-lumen microcatheter—now has the CE mark. The device...