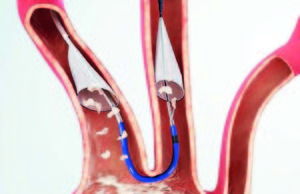
Tag: Sentinel
Investigators find small reduction in disabling stroke from embolic protection device use
Analysis of data from the TVT registry, looking at the impact of cerebral embolic protection dev...
BHF PROTECT-TAVI trial poised to answer key questions about use of cerebral embolic protection during TAVI
Investigators behind the largest randomised trial to date to assess the use of cerebral embolic ...
TCT 2022: No difference in rates of post-TAVI stroke seen with cerebral embolic protection device in PROTECTED TAVR
Among patients undergoing transfemoral transcatheter aortic valve implantation (TAVI) the use of...
Boston Scientific closes acquisition of Claret Medical
Boston Scientific has announced that it has recently closed its acquisition of Claret Medical, which...
Boston Scientific to buy cerebral protection system company
Boston Scientific has signed an agreement to acquire Claret Medical, which developed and commerciali...
ADVERTORIAL: Sentinel Cerebral Protection System provides reassurance to both physicians and patients
Compared with first-generation devices, new-generation transcatheter aortic valve implantation (...
ADVERTORIAL: “All patients” should receive cerebral protection when undergoing TAVI procedures
Data indicate that patients who undergo TAVI with the Sentinel Cerebral Protection System (Claret Me...
Fifty US centres are now using Claret Medical’s Sentinel cerebral protection system
Since it received FDA clearance in June 2017, the Sentinel cerebral protection system (Claret Me...
Claret Medical closes on series C financing of US$14.5m
Claret Medical has announced that it has closed on a series C financing of US$14.5m led by Light...
All-comers data indicate stroke-free survival is improved with Sentinel embolic protection device
Jochen Wöhrle (Department of Internal Medicine II, Cardiology, University of Ulm, Ulm, Germany) ...
US centres of TAVI excellence start to use newly approved Sentinel device
Claret Medical has announced that several of the largest centres of excellence for transcatheter...
Sentinel becomes first cerebral protection device to receive FDA approval
Claret Medical has received regulatory clearance from the FDA for its Sentinel cerebral protecti...
FDA advisory panel recommend de novo approval of Sentinel cerebral protection system
In an open public hearing of the FDA’s circulatory system devices panel, according to a press re...
Meta-analysis indicates embolic protection does reduce stroke after TAVI
In a research letter published in the Journal of the American College of Cardiology, Gennaro Giu...
TCT 2016: SENTINEL trial fails to meet overall primary efficacy endpoint but does in adjusted model
The SENTINEL trial, which was presented at the 2016 Transcatheter Cardiovascular Therapeutics (T...
Claret Medical submits application for FDA approval of its cerebral protection device
Claret Medical has announced its filing of a marketing application with the FDA for clearance of...
TCT 2016: Pivotal trial on cerebral protection device to be presented as a late-breaker
Data from the SENTINEL pivotal IDE trial will be presented during a late-breaking trial session ...
TCT 2016: Late-breakers and first report investigations announced
The Cardiovascular Research Foundation (CRF) has announced the 11 late-breaking trials and 16 fi...