By Max Baghai and Olaf Wendler
The PARTNER-US trial has shown that transcatheter aortic valve implantation (TAVI) is superior to medical treatment in patients with severe, symptomatic aortic valve stenosis who are unsuitable for surgical aortic valve replacement1. In patients at high risk for surgery, the two-year mortality is just above 30% and there is no difference between TAVI and surgery2. Nevertheless, TAVI is less invasive and patients are potentially mobilised earlier, resulting in improved quality of life and cost-effectiveness, which makes it particularly attractive for these patients.
However, recognising that surgery is very safe and cost-effectively done in the UK is important, with an overall perioperative mortality of 2.8% and 5.7% in patients aged ≥80 years3. As perioperative TAVI complications affect outcome, it is thus crucial to control or even eliminate these complications to further improve results.
Since CE mark approval of the first devices in Europe in 2007, it is known that TAVI patients face specific complications, often different from those after surgery. Based on this experience and the PARTNER-US trial, European surgical and cardiology societies recommend the use of TAVI only in patients at least high-risk for surgery4,5. As surgical risk assessment in patients with aortic stenosis is sometimes challenging, the multidisciplinary “Heart Team”, including cardiac specialists with a surgical or interventional background, is crucial and recommended for patient selection6.
The two most used devices in the UK and Europe are the self-expanding CoreValve (Medtronic) and the balloon-expandable Edwards Sapien valve (Edwards Lifesciences)7. Both devices are of 18-French size now, which has reduced the incidence of vascular complications. The main difference between the devices is that while both can be inserted into the heart through a retrograde transfemoral, transaortic or trans-subclavian approach, unlike the CoreValve, the Edwards Sapien can also be inserted through an antegrade transapical access, which makes it technically feasible in almost all patients. Additionally, data from the UK TAVI Registry and the French TAVI Registry show that CoreValve has a higher incidence of atrioventricular blockage (24.4% vs. 7.4%) and paravalvular leakage7. But, patients who undergo transapical TAVI using the Edwards Sapien face higher perioperative mortality, which may be a result of their higher risk profile and/or the added surgical trauma8.
The PARTNER-US trial, which assessed the Edwards Sapien valve, identified stroke and paravalvular leakage as the two main complications after TAVI2. The risk of stroke seems to have less of an effect on the mid-term mortality after two years and will also be addressed in the future by the use of embolic protection devices used in clinical trials at the moment2. Nevertheless, given that the incidence of moderate/severe paravalvular leakage after TAVI is much higher than after surgery (6.9% vs. 0.9%) and also increases two-year mortality, it needs to be addressed before using TAVI in lower-risk patients2. Paravalvular leakage is usually a result of size/anatomy mismatch between the inserted valve and the native aortic annulus.
During the initial experience, over sizing of the inserted valve was an approach to address this issue. Nevertheless, one risks a higher incidence of aortic root trauma and atrioventricular blockage. The cornerstone for a successful TAVI is an appropriate imaging of the left ventricular outflow tract, the native aortic valve and aortic root.
Initially, size of the aortic annulus and anatomy of the aortic valve were assessed by most units before and during TAVI using 2D transesophageal echocardiography (TEE) guidance. Subsequently it was recognised that the aortic root is a 3D structure and that 2D imaging technology can only provide inaccurate data. Therefore an increasing number of TAVI teams started to perform cardiac CT (cCT), providing 3D imaging, allowing for more accurate assessment of the native aortic root.
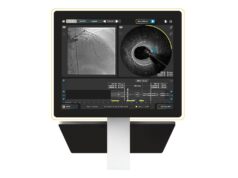
The echocardiography team at King’s was previously involved in the development and trials of 3D TEE, and therefore we used 3D TEE right from the start of our TAVI experience in 20079. 3D TEE does not only provide a three dimensional assessment of the aortic annulus, but even more important, it allows for exact measurements of the left ventricular outflow tract, the aortic root and the sinotubular junction (Figure 1). Size and anatomy of these three anatomical structures are vital for determination of appropriate valve size (Figure 2). This may explain why the incidence of moderate/severe paravalvular regurgitation in our experience of currently more than 200 TAVI procedures compared to published experience7,8 is quite low (7.5%), while aortic root trauma was only observed in 0.5% of patients. In addition to a three-dimensional preoperative cCT assessment, 3D TEE provides online information immediately before and after valve deployment, vital for the detection of causes of ventricular failure after valve deployment. Although 3D TEE is more investigator dependent, it has been shown to provide as accurate data as cCT10.
Given the growing evidence that paravalvular leakage affects mid-term survival, quantifying and qualifying of paravalvular leakage after TAVI is an additional key strength of 3D TEE. It allows decisions on additional treatment such as repeat balloon dilation of the inserted valve or implantation of a second valve. In addition, 3D TEE provides accurate imaging support in patients with less calcification of the native aortic valve or previous implanted aortic but also mitral bioprostheses, where fluoroscopy is of less value to determine exact position of the valve during deployment.
Apart from these technical issues during TAVI, future development of transcatheter heart valve devices and techniques will make it easier to prevent this life-threatening complication. Therefore, it is not surprising that future trials on patients with lower-risk for surgery are being conducted at present. Results of the PARTNER-US II trial, including patients with moderate-risk for surgery, are expected next year and the UK TAVI trial is close to recruiting patients.
Max Baghai and Olaf Wendler, Department of Cardiothoracic Surgery, King’s College Hospital/King’s Health Partners, London, UK
Olaf Wendler is a proctor for the Edwards Lifesciences transcatheter heart valve programme, and has worked as a consultant for St Jude Medical and JenaValve
References
1. Makkar et al. N Engl J Med 2012; 366:1696–1704
2. Kodali et al. N Engl J Med 2012; 366:1686–95
3. 6th National adult cardiac surgical database report 2008, Society for Cardiothoracic Surgery in Great Britain and Ireland.
4. Holmes et al. J Am Coll Cardiol 2011; 58:445–55.
5. Vahanian et al. European Heart Journal 2008; 29:1463–70
6. Naber et al. EuroIntervention 2012; 7:1257–74
7. Moat et al. JACC 2011; 58:2130–38
8. Thomas et al. Circulation 2011; 124:425–33
9. Bhan et al. Eur Heart J 2008; 29:733–900
10. Ng et al. Circ Cardiovasc Imaging 2010; 3:94–102