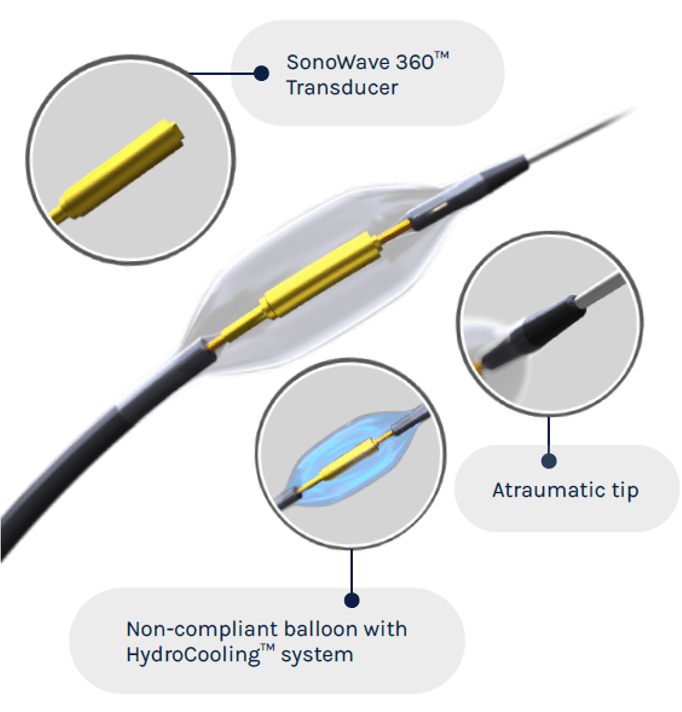
 Investigators have completed enrolment in the RADIANCE-II pivotal trial of the Paradise ultrasound renal denervation (uRDN, Recor Medical) system for the treatment of uncontrolled hypertension.
Investigators have completed enrolment in the RADIANCE-II pivotal trial of the Paradise ultrasound renal denervation (uRDN, Recor Medical) system for the treatment of uncontrolled hypertension.
Subject to study outcomes, the RADIANCE-II results will be combined with the previously released data from the company’s RADIANCE-HTN SOLO and TRIO studies in a premarket application to the US Food and Drug Administration (FDA), and will be presented to the scientific and medical communities in publications and conferences later this year.
The RADIANCE-II pivotal trial is a randomised, sham-controlled clinical trial of Recor’s Paradise uRDN system for the treatment of uncontrolled hypertension in patients on zero-to-two antihypertensive oral medications.
After four weeks of washout from antihypertensive medications, patients are randomized at a 2:1 ratio to either Paradise uRDN or a sham procedure. The primary efficacy endpoint is the difference in daytime ambulatory systolic blood pressure between Paradise uRDN and sham measured at two months post-procedure, while the primary safety endpoint is a composite of 30-day major adverse events.
More than 1,000 study subjects have been enrolled over three years at more than 50 study centres in six countries, with over 200 patients randomised to uRDN or a sham procedure.
“The RADIANCE-II pivotal trial was carefully designed to assess the BP-lowering efficacy and safety of treating uncontrolled hypertension with the Paradise uRDN System. We are grateful to the investigators and study participants across the globe for their work on the study—especially through the challenges created by the COVID-19 pandemic over the past two years. We look forward to their continued help in the coming months to complete this landmark study, and we are excited to assess trial outcomes later this year,” said co-principal investigators Michel Azizi (Université Paris Cité, Paris, France) and Ajay Kirtane (Columbia University, New York, USA).
RADIANCE-II is the third component of Recor’s RADIANCE clinical trial programme in patients with hypertension, which includes the RADIANCE-HTN SOLO and TRIO studies. In the randomised and sham-controlled SOLO trial, 146 patients with mild-to-moderate hypertension were assessed in an “off-meds” setting. In the landmark TRIO trial, 136 patients who remained hypertensive, despite a standardised triple antihypertensive therapy, were randomised 1:1 to the Paradise uRDN treatment or sham.
Both studies met their primary effectiveness endpoints. ReCor has begun the fourth component of their RADIANCE programme with the launch of the Global Paradise System (GPS) registry—a real-world study of patients with uncontrolled hypertension initiated in Germany earlier this year.
The Paradise uRDN system bears the CE mark for the treatment of hypertension in Europe and is an investigational device in the USA. Hypertension is the leading contributor to disease burden worldwide, leading to increased cardiovascular morbidity and mortality, poorer quality of life, and increased cost to health systems.