This advertorial is sponsored by Osprey Medical
There is a growing recognition of the risk of contrast-associated acute kidney injury (AKI) to patients undergoing coronary interventions in the cath lab, as well as the strategies that can be employed to limit the damage in those at the most risk. That is according to Carlo Briguori, chief of the Laboratory of Interventional Cardiology at Mediterranea Cardiocentro in Naples, Italy, who spoke to Cardiovascular News about the need for vigilance over preventing AKI, effective treatment of acute coronary syndrome (ACS) patients at high risk for kidney damage, and his experience with the DyeVert Contrast Reduction System (Osprey Medical), which is designed to help limit patients’ exposure to contrast media—a major cause of kidney damage during invasive coronary procedures.
“In the past, interventional cardiologists did not have such a good awareness of this risk, and were much more concentrated on treating the coronary [artery], or the heart,” says Briguori, discussing the understanding of the risks faced by patients with chronic kidney disease (CKD). “Today, I think that the majority of interventionalists have a good knowledge of the risk to the kidneys and the poor outcomes, so they are performing preventative strategies.”
For the majority of patients who visit the cath lab, the risk of AKI is low. However, there is a subset of patients, particularly those with CKD, who are at the greatest risk of harm. Briguori comments: “In general, if you think about all of the people undergoing coronary interventions, including coronary angiography or angioplasty, globally the risk of AKI may be considered low, between 1‒5%. But, if you look specifically at a subgroup of patients with baseline CKD, which nowadays is roughly 30% of the population, the risk is increased, and may be up to 10‒13%.”1

AKI is associated with increased hospital length of stay, increased 30-day readmissions, and poor clinical and economic outcomes for patients2, meaning that identification of those at the most risk, prior to the formation of any treatment plan, is fundamental to preventing the potential adverse effects of kidney damage. Briguori explains that there are a number of predictors that can point towards an elevated risk of AKI during a coronary procedure, which include age, the presence of diabetes, CKD, anaemia, and haemodynamic instability.
One of the major contributors to kidney damage during a coronary intervention is over-exposure to contrast media used to improve contrast resolution during angiographic imaging. This can lead to contrast-associated AKI, which is an independent predictor of major adverse renal and cardiovascular events.
The rate of AKI is commonly defined as a serum creatinine (sCR) increase ≥0.3 mg/dl within 72 hours of exposure to contrast media, and can be stratified according to criteria set out by the Acute Kidney Injury Network3, which offers a staged assessment of kidney damage based upon the severity of sCR increase following a procedure. For the most severe cases, the worst outcome can be dialysis, explains Briguori. “Dialysis is of course a very bad complication for the patients, but also for the physician performing the coronary procedure, so we do not want to have any patients going on dialysis, even the patients at high risk,” he comments.
Strategies to limit kidney damage
Strategies to limit kidney damage in these high-risk patients, historically have focused on management of hydration through saline infusion—which can be tailored to the patient based upon characteristics such as left ventricular end diastolic pressure4, or urine flow rate.5 However, according to Briguori, this approach is more challenging in an acute setting, where there may not be time to appropriately tailor the required level of hydration to the needs of the patient. He says: “If I have a patient coming into the cath lab for acute myocardial infarction (MI), I cannot wait one hour to hydrate—I need to go into the cath lab immediately. If the patient is anaemic, diabetic, or has CKD, it is important, but I cannot wait to do optimal hydration because there is an acute MI.”
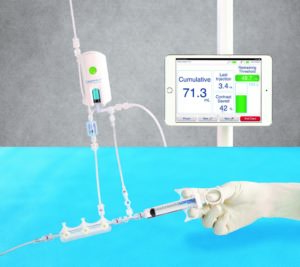
A second important strategy is to limit the patient’s exposure to contrast media. This is where the DyeVert system comes into play. The system is designed to reduce contrast media delivered to the patient while maintaining image quality during a procedure, as well as shot-by-shot monitoring of the contrast media volume that is administered to the patient. DyeVert is a single-use, sterile device connected by a manual injection syringe, allowing modulation of contrast media during injections. A portion of the injected contrast media is diverted through a secondary fluid pathway controlled by a pressure-compensating diversion valve during an injection, allowing a decrease in over-injection of contrast media and less aortic reflux.
Briguori has been at the forefront of investigating the effectiveness of DyeVert, leading the first major pivotal study of the device, findings of which were published in the journal Catheterization and Cardiovascular Interventions in 20205. The single-centre study assessed whether use of the DyeVert system reduced the AKI rate in ST-elevation myocardial infarction (STEMI) and high-risk Non-ST-elevation myocardial infarction (NSTEMI) patients undergoing invasive coronary procedures in the Interventional Cardiology Unit at Mediterranea Cardiocentro, Napoli, Italy between 2017 and 2019. A total of 180 patients were analysed in two groups, 90 patients undergoing a coronary intervention assisted by the DyeVert system were compared to a control group of 90 patients in whom contrast media was regulated using a manual injection syringe.
Briguori and colleagues found that contrast media volume was higher in the control group than in the DyeVert group (130ml vs. 99ml; p<0.001), with AKI occurring in seven patients (8%) in the DyeVert group and in 17 patients (19%) in the control arm (odds ratio, 0.37; p=0.047). Overall, the study found that contrast media volume was reduced by 38% among those for whom DyeVert was employed, compared to patients in whom contrast media was deployed manually. A further finding was a 25% reduction in hospital length of stay in the DyeVert group compared to those whose procedures did not involve the device. “What this translates to is a clinical advantage,” says Briguori, commenting on the findings. “We have found an important reduction in AKI, in the subgroup with DyeVert, which means that we have an important impact on kidney damage prevention.”
He adds: “The device has been demonstrated to reduce contrast volume in the procedure up to 40%.7 That means if we inject 100ml, actually we then save 40ml, and the real contrast volume injection to the patient is 60ml, which is really important in high-risk patients. Limiting contrast media volume by 40% will translate into an important reduction of the AKI events in the cath lab.”
Further study of the system is taking place in the REnal Insufficiency Following Contrast MEDIA Administration TriaL IV (REMEDIAL IV) randomised trial, in which Briguori is the principal investigator, taking place across six Italian centres. Around half of the trial’s 400 patients have been recruited, randomised 1:1 to undergo the DyeVert assisted procedure, or contrast by normal manifold—with enrolment expected to be concluded in 2021.Briguori comments that the trial will provide important insights into the potential for the widespread use of the DyeVert system in cath labs, and its particular importance for the patients at risk of AKI.
Carlo Brigouri is chief of the Laboratory of Interventional Cardiology at the Clinica Mediterranea, Naples, Italy and co-director of clinical research at the Laboratory of Interventional Cardiology, Vita e Salute San Raffaele University Medical School, Milan, Italy.
References