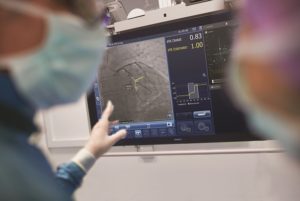
Philips has announced a new randomised controlled trial to assess percutaneous coronary intervention (PCI) guided by its coregistration platform, which combines data of an instant wave-Free Ratio (iFR) measurement and an angiogram, compared with standard of care treatment guided by an angiogram alone.
European and US guidelines already endorse the use of coronary physiologic measurements with iFR and fractional flow reserve (FFR) for diagnostic purposes to determine the significance of a narrowed coronary artery. A company statement outlines that the DEFINE GPS (Distal evaluation of functional performance with intravascular sensors to assess the narrowing effect: guided physiologic stenting) study will be the first time that the use of iFR in conjunction with the Philips Image-Guided Coregistration System (SyncVision) is evaluated for PCI guidance and the optimisation of treatment outcomes.
Under the current standard of care in PCI, clinicians navigate a balloon catheter and coronary stent to the treatment area using interventional X-ray guidance (coronary angiography). In the study, an iFR pullback measurement, which uses pressure wires to map the pressure profile of the disease distribution along the length of the affected vessel, will be overlaid on the X-ray image to guide treatment. iFR is a next-generation physiologic measurement that uses the same pressure guide wires and equipment as FFR but avoids the administration of hypaeremic agents to patients.
Principal investigator of the DEFINE GPS study Allen Jeremias, (St. Francis Hospital, Roslyn, New York, and Cardiovascular Research Foundation, New York, USA) says in the press release: “PCI has made a major positive impact on many coronary artery disease patients’ lives. However, when we look back at all the major, high-quality stent trials over the past 20 years we see that around 20–30% of patients continue to have recurring chest pain at one year after receiving treatment. With the DEFINE PCI study, we observed that the current approach to PCI has limitations for identifying the locations of physiologically significant arterial lesions. With DEFINE GPS we will be able to determine if a physiology-based PCI approach results in superior patient outcomes when compared with standard angioplasty.”
“As coronary stenting is applied to increasingly complex patients, it is essential that we ensure that all segments of coronary artery disease that need treatment are treated, a process that we believe can be facilitated by iFR assessment of the entire coronary artery, coregistered to the angiogram.” says Gregg W Stone (Icahn School of Medicine at Mount Sinai, New York, USA), chairman of the DEFINE GPS trial. “We are thrilled to be able to examine the extent to which this technique improves patient outcomes in the large-scale DEFINE GPS trial.”
The global multicentre, prospective, randomised controlled DEFINE GPS study will investigate the impact of iFR coregistration on both outcomes and cost effectiveness in up to 3,000 participants at 75 sites. The primary endpoint is target vessel failure (a composite of cardiac death, target vessel myocardial infarction, and ischaemia-driven target vessel revascularisation) or rehospitalisation for progressive or unstable ischaemia at two years.
Chris Landon, senior vice president and general manager image guided therapy devices at Philips, states in the press release: “iFR continues to be adopted into clinical practice, with mounting evidence that this innovative technology contributes to improving outcomes, reducing costs and enhancing the patient experience. This major study will provide a definitive answer to the question of the overall improvement resulting from the use of a functional guidance strategy on patient outcomes and cost.”
The DEFINE GPS study is sponsored by Philips with the Cardiovascular Research Foundation overseeing core lab and clinical event committee activities. The first patients will be recruited in the second half of 2020.
The company says that the Philips Image-Guided Coregistration System (SyncVision) is part of Philips’ unique portfolio of systems, smart devices, software and services in image-guided therapy, which combine to provide healthcare providers with sophisticated, procedure-oriented solutions.