Royal Philips today announced the introduction of the 160cm US Food and Drug Administration (FDA)-approved version of its LumiGuide endovascular navigation wire. This enhanced LumiGuide guidewire, which utilises the company’s Fiber Optic RealShape (FORS) technology, was used for the first time on a patient by Carlos Timaran (UT Southwestern Medical Center, Dallas, USA). He used the technology during a complex aortic aneurysm repair operation, marking the 1000th patient treated milestone using FORS since its first clinical use in 2020.
A press release notes that the introduction of a longer FORS-enabled LumiGuide navigation wire enables US clinicians to visualise a broader range of catheters and expands usage of this technology to patients in the USA treated in centres equipped with LumiGuide.
“The new enhanced Philips LumiGuide navigation guidewire represents a significant leap forward in endovascular surgery, offering unprecedented 3D visualisation and precision during complex procedures,” said Timaran. “As the first to use this guidewire for a complex aortic repair, I experienced firsthand its potential to revolutionise how we approach minimally invasive vascular interventions. The patient who underwent this groundbreaking procedure is doing well, further validating the efficacy of this innovative technology.”
Philips shares that LumiGuide—powered by the company’s FORS technology—allows clinicians to see their guidewires and catheters in 3D and colour as they manipulate them inside the patient’s body, from any angle, in real-time, and with minimal radiation. The company adds that its FORS technology simplifies navigation in tortuous vessels.
Using this advanced technology, research published in the Journal of Vascular Surgery and from Philips shows that complex cases such as aortic repair procedures can be done 37% faster and use 70% less X-ray imaging during the process.
Providing greater reach and enhanced catheter loading possibilities than the company’s existing 120cm (47 inch) guidewire, Philips claims that its new 160cm (63 inch) LumiGuide wire enables US physicians to experience 3D device guidance with more catheters than before, enabling use of the technology for more patients and procedures.
“LumiGuide unlocks the colour visualisation of wires, catheters, and patient anatomies in 3D from any angle, including simultaneous angles to generate ‘virtual biplane’ images. Combined with device navigation viewed from angles physically unachievable using conventional C-arm systems, it has already been shown to improve workflows, reduce procedure times, and decrease patient and staff radiation dose,” said Atul Gupta, chief medical officer for diagnosis and treatment at Philips and a practicing interventional radiologist.
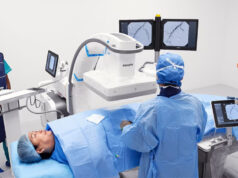
Philips advises that LumiGuide integrates seamlessly into the company’s image-guided therapy system, Azurion, allowing its use alongside preoperative cross-sectional imaging.