Data from the EXPAND G4 study, evaluating the performance of the latest generation MitraClip (Abbott) device—the Mitraclip G4—demonstrate that the device has a similar level of procedural effectiveness, without an increase in adverse events.
These were the conclusions presented by Bassem Chehab (Kansas Heart Hospital, Wichita, USA) who presented the data during a late-breaking trial session at PCR Valves e-course (22–24 November, virtual). Detailing the developments in the device, Chehab noted that the fourth generation MitraClip G4 system features four clip sizes, with the option for independent leaflet grasping through a controlled gripper actuation, an improved clip deployment sequence and continuous left atrial pressure monitoring to enable ease of implantation.
The EXPAND G4 study, a prospective, multicentre, single arm, post market, real-world observational study, looked at 101 consecutive consented patients receving the MitraClip at US 14 sites followed through baseline, discharge and 30 days.
Key outcome measures included MR severity, major adverse events (MAE), survival, procedural outcomes, quality of life assessed using KCCQ and functional capacity assessed using the New York Heart Association (NYHA) functional class. Data were adjudicated using an echocardiographic core laboratory (ECL).
The primary objective of the study was to report on core lab-adjudicated 30-day real-world outcomes with the G4 system, which was compared against data from the EXPAND study, which assessed the MitraClip (NTR/XTR) system. Patient demographics were similar between the EXPAND G4 and EXPAND populations, Chehab commented, noting that there were differences in mitral valve complexity, which was more common in the EXPAND G4 study, and less primary MR compared to EXPAND.
The number of clips per subject was around 1.4 per patient in EXPAND G4, compared to 1.5 in EXPAND. The majority (45%) of clips were NTW (a wider clip size). NT clips were used in 28% of cases, XTW In 24% and XT in only 3%. Chehab explained that 87% were treated with at least one wider clip, and 60% were treated with NTW alone followed by another clip.
Regarding the combination of the clips, Chehab explained that 61% had one clip versus 38% with two, while less than 1% had a three-clip combination. In patients who had a single clip mix, 82% had wider clips, with 59% of these being the NTW. Regarding the double-clip mix, he explained, the first clip was primarily a wider clip, 53% being the NTW and 39% being the XTW, but as a secondary clip the primary clip of choice was the NT at 66%.
“The number of patients who had one clip was 61% with EXPAND G4 and that is a definite change from the 59% treated with the one clip in the EXPAND study by itself,” he said.
In terms of outcomes, Chehab commented that the implant rate, procedural time and hospital length of stay were “on par” with the equivalent data from EXPAND study. Of note, he said, was the higher acute procedural success rate in EXPAND G4—99% compared to 95.9% in EXPAND—while device time and fluoroscopy time were also reduced.
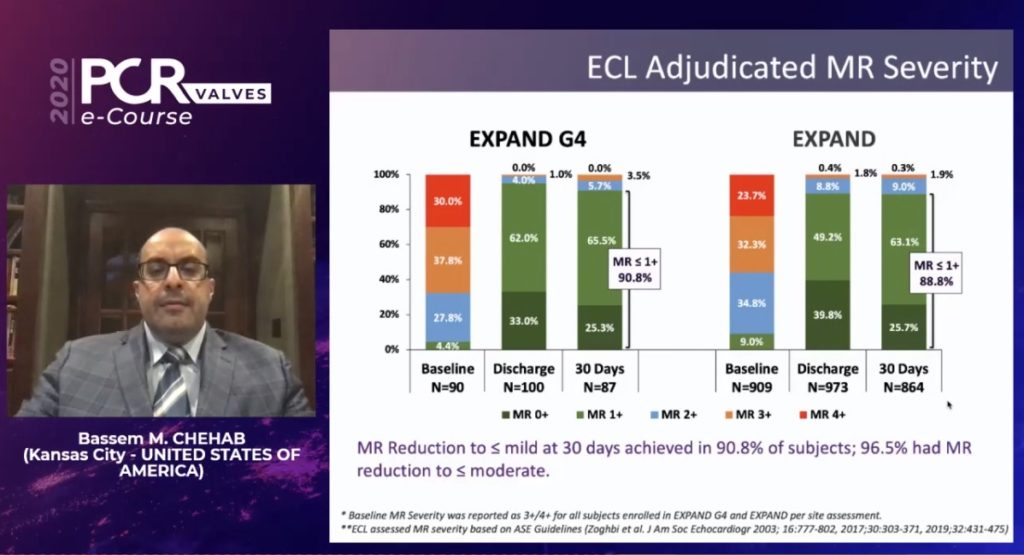
Regarding the echo core lab adjudicated MR severity, at 30 days, patients who had MR reduction to mild or less were 90.8% of the subjects, compared to 88.8% in the EXPAND study. “This is the difference between EXPAND G4 and EXPAND,” Chehab commented. There was also a statistically significant change in the NYHA class and the KCCQ at 30 days, he added.
Looking at 30-day adverse events, all-cause death was seen in 2% of patients, compared to 2.3% in EXPAND, and there were no recorded incidences of myocardial infarction, stroke or ischaemic events. Regarding the mitral valve gradient, Chehab said that although wider clips were used more frequently in EXPAND G4, there was no observation of increased mitral valve gradient post procedurally compared to the third generation clip sizes.
“Compared to the third generation MitraClip NTR and XTR systems, the introduction of the two new additional clip sizes and independent grasping with the MitraClip G4 system has resulted in similar procedural effectiveness—almost 91% of patients had mild or less MR, versus 88.9% with the older systems.
“More patients were treated with just one clip, at 61% with the G4 versus 55% with the NTR and the XTR. There was faster device time, with 39 minutes with the G4 versus 46 minutes with the NTR and XTR.
“Clearly [there is a] change, and constant improvement and good effectiveness in the technology. These procedural enhancements between the two systems were obtained without any increase in adverse events, which is a pretty important outcome in this study.”










