Otsuka Medical Devices and ReCor Medical, a subsidiary of Otsuka, announce the filing of the pre-market approval (PMA) application to the US Food and Drug Administration (FDA) for the Paradise ultrasound renal denervation system (uRDN) system in the treatment of uncontrolled hypertension.
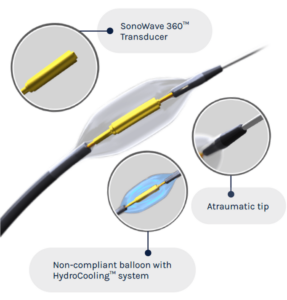
The Paradise uRDN system is designed to reduce sympathetic nerve activity by denervating nerves which surround the renal arteries with the goal of reducing blood pressure, and uses a combination of ultrasound energy to denervate the renal nerves and a water-filled balloon to protect the renal artery. The system employs an interventional procedure in which a catheter is placed in each of the main renal arteries, following which two to three seven-second ultrasound emissions are delivered to denervate the surrounding renal nerves, thereby reducing blood pressure.
Since 2009, ReCor has been focused on developing and testing the Paradise uRDN system to treat hypertension safely and effectively. ReCor has three global, independently powered, sham-controlled randomised clinical trials of the Paradise uRDN system in more than 500 patients with uncontrolled hypertension: RADIANCE-HTN SOLO, RADIANCE-HTN TRIO and RADIANCE II. Each RADIANCE trial met its prespecified primary efficacy endpoint of blood pressure reduction, with positive safety.
RADIANCE II is the USA FDA’s investigational device exemption (IDE) pivotal trial. In September 2022, ReCor and Otsuka Medical Devices announced that the trial successfully reached its primary efficacy endpoint. Results showed a reduction in daytime systolic ambulatory blood pressure of -7.9 mmHg in those treated with uRDN and a difference between uRDN and sham of -6.3 mmHg (p <0.0001). The results from the all three RADIANCE clinical trials have been included in the submission for approval to the FDA.
The uRDN system bears the CE mark for the treatment of hypertension in Europe and is an investigational device in the USA and Japan.










