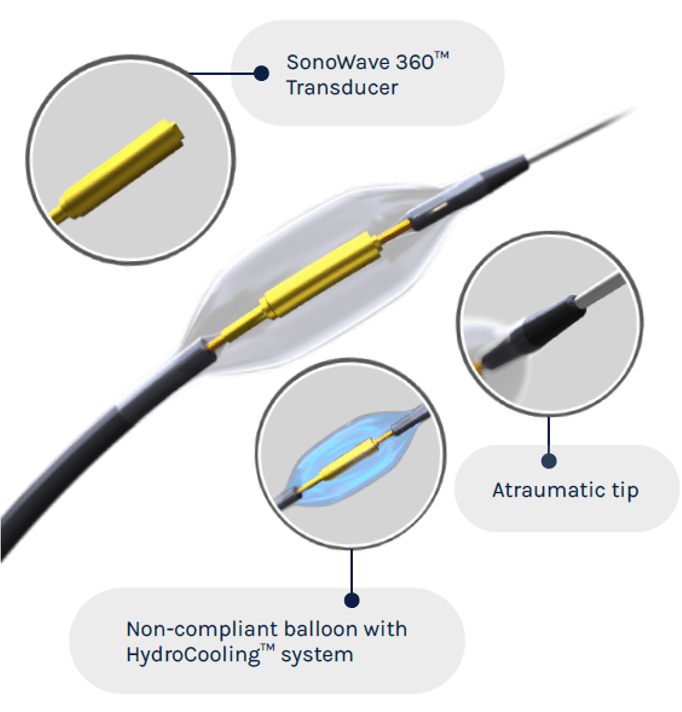
 ReCor Medical and Otsuka Medical Devices have announced that the RADIANCE II US Food and Drug Administration (FDA) investigational device exemption (IDE) pivotal trial evaluating the Paradise ultrasound renal denervation (uRDN) system as a treatment for hypertension met its primary efficacy endpoint, demonstrating a statistically significant reduction in daytime ambulatory systolic blood pressure between treatment and a sham procedure measured at two months.
ReCor Medical and Otsuka Medical Devices have announced that the RADIANCE II US Food and Drug Administration (FDA) investigational device exemption (IDE) pivotal trial evaluating the Paradise ultrasound renal denervation (uRDN) system as a treatment for hypertension met its primary efficacy endpoint, demonstrating a statistically significant reduction in daytime ambulatory systolic blood pressure between treatment and a sham procedure measured at two months.
The RADIANCE II US FDA IDE pivotal trial is a randomised, sham-controlled clinical trial of the ReCor Paradise uRDN System for the treatment of patients with uncontrolled hypertension. 224 patients with mild-to-moderate uncontrolled hypertension, previously treated with up to two medications, were randomised while off medications at more than 60 study centres in eight countries.
“Despite the truly formidable challenges of conducting a complex clinical trial in the throes of the COVID-19 pandemic, we are thrilled to observe these positive results of RADIANCE II, especially in light of those we have previously reported from RADIANCE-HTN SOLO and TRIO,” said study principal investigators Ajay Kirtane (Columbia University, New York, USA) and Michel Azizi (Hôpital Européen Georges Pompidou, Paris, France). “We cannot adequately convey our thanks to the patients, coordinators, and study physicians for their collective efforts, and we very much look forward to being able to present and publish the complete study details in the near future.”
“We at Otsuka are very pleased with the positive outcome of the RADIANCE II study,” said Kazumichi Kobayashi, executive deputy president of Otsuka Medical Devices. “With three successful clinical trials of the Paradise uRDN system, we believe even more strongly that the Paradise system can become an important treatment option for patients and physicians struggling to control blood pressure.”
“ReCor is thrilled that the RADIANCE II trial met its primary efficacy endpoint. Following the positive SOLO and TRIO clinical trials, RADIANCE II adds to the evidence for the Paradise system as a potential future treatment for patients with uncontrolled hypertension,” said ReCor president and CEO, Andrew M Weiss. “We would like to express our gratitude to the principal investigators, steering committee and all investigators for their efforts throughout this important trial.”
RADIANCE II is the third and largest component of ReCor’s RADIANCE Global Program—randomised and sham-controlled studies evaluating the Paradise uRDN System in patients with hypertension. The first two studies in the series are the previously reported RADIANCE-HTN SOLO (conducted in patients with mild-to-moderate hypertension) and TRIO (conducted in those who remained hypertensive despite being on antihypertensive therapy). Both studies met their primary effectiveness endpoints.













