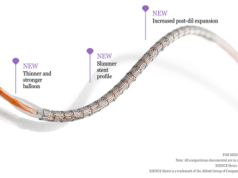
Abbott has announced that Japan’s Ministry of Health Labour and Welfare (MHLW) has granted national reimbursement for Xience Sierra, the newest generation of the company’s everolimus-eluting coronary stent. According to a press release, the stent improves upon previous versions of Xience with an enhanced stent design, a new delivery system, and unique sizes to help doctors treat challenging cases.
The press release reports that the Xience Sierra was designed to help doctors more easily treat people with difficult-to-treat blockages that involve multiple or totally blocked arteries or complications such as diabetes. It adds that national reimbursement in Japan will enable doctors to treat more patients with the stent through the country’s health insurance plans. Xience Sierra was approved in Japan on 4 April 2018, received CE mark in Europe late last year, and is under review with the US Food and Drug Administration.
Chuck Brynelsen, senior vice president of Abbott’s vascular business, comments: “Extensive clinical data and 10 years of real-world experience with the Xience family of stents provide doctors with confidence that they are treating their patients with one of the safest stents available. National reimbursement of Xience Sierra will provide people in Japan with greater access to this life-changing technology that can help them live their best lives.”
The press release notes that Xience has been studied in over 100 clinical trials and in 10 years of global real-world experience. Its safety profile is unprecedented with consistent low rates of stent thrombosis, even in complex cases. More than eight million people worldwide have received a form of the stent since its initial regulatory approval.