Innovative Cardiovascular Solutions (ICS) has announced the first clinical use of its next-generation Emblok embolic protection system in patients undergoing transcatheter aortic valve implantation (TAVI) procedures in its European feasibility study. The cases were successfully performed by primary investigator Federico De Marco (Policlinico San Donato, Milan, Italy).
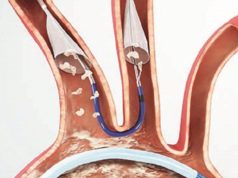
The Emblok system is designed for use in TAVI and other structural heart procedures, which are associated with an increased risk of neurological events compared to conventional surgery due to embolic debris that is liberated throughout the procedure. A press release reports that the next-generation Emblok system is the first embolic protection system to fully protect the cerebral, abdominal and peripheral vasculature with complete circumferential aortic coverage to capture and remove the liberated embolic debris throughout the entire TAVI procedure.
In addition to offering protection to more patients, according to the press release, the next-generation Emblok system provides protection throughout the entire TAVI procedure. The embolic filter deploys before the TAVI catheter enters the aorta and remains deployed until the TAVI catheter is removed.
De Marco comments: “I am excited to have one embolic protection system I can use across nearly all my TAVI patients to provide complete protection during the entire TAVI procedure. There is nothing like it on the market.”
R Kevin Plemmons, CEO of ICS, says: “The prior generation demonstrated excellent performance, however we saw an opportunity to modify the device dimensions to be suitable for even more patients. The next-generation Emblok system is appropriate for 97% of all TAVI patients, whereas other commercial embolic protection devices may not be suitable for all anatomies.”
The entire system is 11F and allows both the embolic filter and integrated pigtail catheter to be deployed through a single femoral access site. The Emblok system is available for investigational use only and is not approved for sale. The company plans to begin its US early feasibility study later this year. Study endpoints will include acute cerebral embolic burden and major adverse cardiac and cerebrovascular events (MACCE) at 30 days.