ReCor Medical and its parent company Otsuka Medical Devices have welcomed the release of updated and expanded recommendations for the management of arterial hypertension issued by the European Society of Hypertension (ESH).
Presented during the ESH’s 32nd annual European meeting on hypertension and cardiovascular protection (24–26 June, Milan, Italy) and published in the Journal of Hypertension, the new guidelines are designed to serve as “an essential resource” for healthcare professionals treating individuals with hypertension, according to ESH.
The 2023 ESH Guidelines for the Management of Arterial Hypertension, endorsed by the European Renal Association (ERA) and the International Society of Hypertension, include recommendations for the use of renal denervation, which can be considered, as an additional treatment option for patients with resistant hypertension on three or more antihypertensive medications, as a treatment option for patients with uncontrolled hypertension despite the use of antihypertensive drug combination therapy, or if a patient’s drug treatment elicits serious side effects and poor quality of life.
“Recommendation of renal denervation by the recently published ESH 2023 guidelines is a significant milestone for the field of arterial hypertension management. The decision to consider renal denervation as a treatment option is based on the demonstrated efficacy and safety of the procedure in the published results of several rigorous randomised and sham-controlled trials,” said Michel Azizi (Université Paris Cité, Hôpital Européen Georges Pompidou, Paris, France). “The recommendations state that renal denervation should be performed in experienced specialised centres, and that the process of patient selection should be done by a multidisciplinary team.”
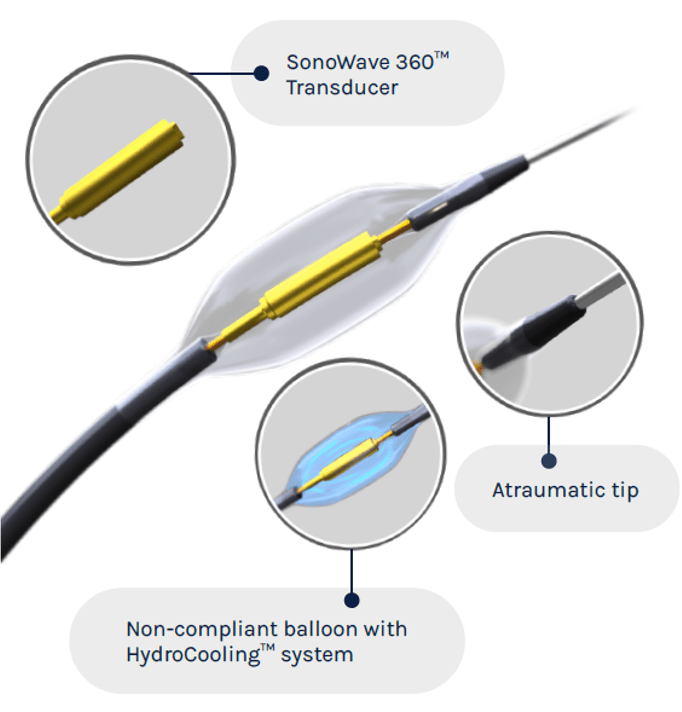
ReCor Medical has completed three global, independently powered, sham-controlled randomised clinical trials of the Paradise ultrasound renal denervation system in more than 500 patients with uncontrolled hypertension. The RADIANCE-HTN SOLO, RADIANCE-HTN TRIO and RADIANCE II clinical studies met their prespecified primary efficacy endpoints of blood pressure reduction, with positive safety outcomes.
“The ESH guidelines support the experience of European clinicians who are already using the Paradise ultrasound renal denervation system for some patients who need an additional treatment option to achieve meaningful reductions in blood pressure,” said ReCor president and CEO, Lara Barghout. “The urgent global need to address the burden of uncontrolled hypertension remains, and we are committed to revolutionising the way hypertension is treated and bringing the benefits of ultrasound renal denervation to patients who need it. In the coming months, we will work with the US Food and Drug Administration (FDA) to complete their review of our premarket approval seeking approval of ultrasound renal denervation in the USA.”
In November 2022, ReCor Medical announced its submission of a premarket approval application for the Paradise ultrasound renal denervation system to the US FDA. The FDA Circulatory Systems Devices Panel of the Medical Devices Advisory Committee will hold a meeting virtually on 22‒23 August, to discuss, make recommendations and vote on devices indicated to reduce blood pressure in patients with hypertension.
The Paradise ultrasound renal denervation system bears the CE mark for the treatment of hypertension in Europe and is an investigational device in the United States and Japan.