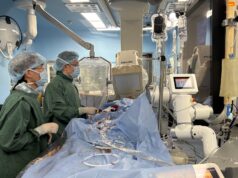
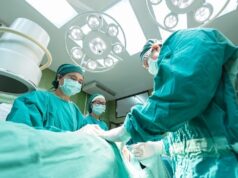
The first US patient has been enrolled in Mitralign’s SCOUT study, which is evaluating the company’s percutaneous tricuspid valve annuloplasty system (Trialign). The procedure was performed by the study’s principal investigator Rebecca Hahn (director of Interventional Echocardiography, NewYork-Presbyterian/Columbia University Medical Center, New York, USA) and by Susheel Kodali (director, Structural Heart and Valve Program at NewYork-Presbyterian/Columbia University Medical Center, New York, USA).
SCOUT is a US- based early feasibility investigational device exemption study using the Trialign system in patients with symptomatic chronic functional tricuspid regurgitation. It will assess the early safety and feasibility of the device for the treatment of tricuspid regurgitation in patients with a minimum of moderate tricuspid regurgitation and in whom left”sided valve surgery is not planned.
Hahn says: “We are extremely excited to be pioneering a novel solution in percutaneous repair for the tricuspid valve. Given the reports that operative mortality for tricuspid valve replacement surgery can top 30%, coupled with the lack of treatment options, this system represents a very welcome advancement.”
Hahn will be writing about the SCOUT trial and the Trialign system in a forthcoming issue of Cardiovascular News.