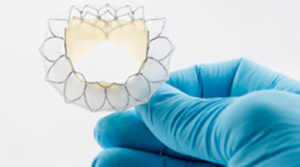
The FDA has given Neovasc approval for participating physicians to treat patients with the company’s 40mm Tiara valve in its TIARA-I early feasibility trial. The trial is a multinational, multicentre study—being conducted at centres in the USA, Europe and Canada—that aims to assess the safety and performance of Neovasc’s Tiara mitral valve system and implantation procedure in high-risk surgical patients with severe mitral regurgitation.
A press release reports that the Tiara device is a self-expanding mitral bioprosthesis that is specifically designed to treat mitral valve regurgitation by replacing the diseased valve. It adds that conventional surgical treatments are only appropriate for about half of patients with mitral regurgitation (who number an estimated four million in the USA alone). The valve is implanted in the heart using a minimally invasive, transapical transcatheter approach and is designed to replace the diseased native mitral valve without the need for open-heart surgery or use of a cardiac bypass machine.
Alexei Marko, chief executive officer of Neovasc, says: “We believe the addition of the 40mm size is an important step in the Tiara programme and should significantly increase the number of patients eligible for treatment. With both the 35mm and 40mm sizes now available, we are continuing development activities to bring additional sizes into clinical use.”
![]()










