This advertorial is sponsored by PIE Medical

Findings of the multicentre FAST II clinical trial, presented virtually at EuroPCR 2021 (18–21 May, virtual) underscore the potential of CAAS vessel fraction flow reserve (vFFR, PIE Medical) software—a non-invasive, angiography-based method for calculating fractional flow reserve (FFR) values—as a faster and easier physiological lesion assessment tool, compared to invasive, wire-based techniques.
This was among the take-home messages presented by Joost Daemen (Department of Cardiology, Thoraxcenter, Erasmus University Medical Center, Rotterdam, The Netherlands), principal investigator of the prospective, six-centre observational Fast Assessment of STenosis severity (FAST) II trial. Daemen presented the results during a late-breaking trial session at the EuroPCR meeting.
Traditional FFR utilises a specialised guide wire to measure pressure differences across a coronary stenosis. CAAS vFFR is an alternative, angiographic method used to assess coronary physiology and calculate FFR values, indicating the significance of specific coronary lesions and showing where blood flow is limited.
During his presentation, Daemen told EuroPCR attendees that while FFR-guided percutaneous coronary intervention (PCI) is superior to angiography-guided PCI, the clinical uptake of the this technique remains low. This is in part, he said, is due to the perceived additional procedural time and cost with which it is associated, as well as a potential discomfort to patients due to the administration of hyperemic agents.
“The problem with physiological lesion assessment is the fact that at present you are forced to use dedicated pressure wires or microcatheters that are not necessarily the best, most user-friendly wires,” Daemen told Cardiovascular News, discussing the presentation. “Moreover these devices come at a specific cost—and if you want to rely on hyperemic indices you are forced to use hyperemic agents which go along with specific patient discomfort.”
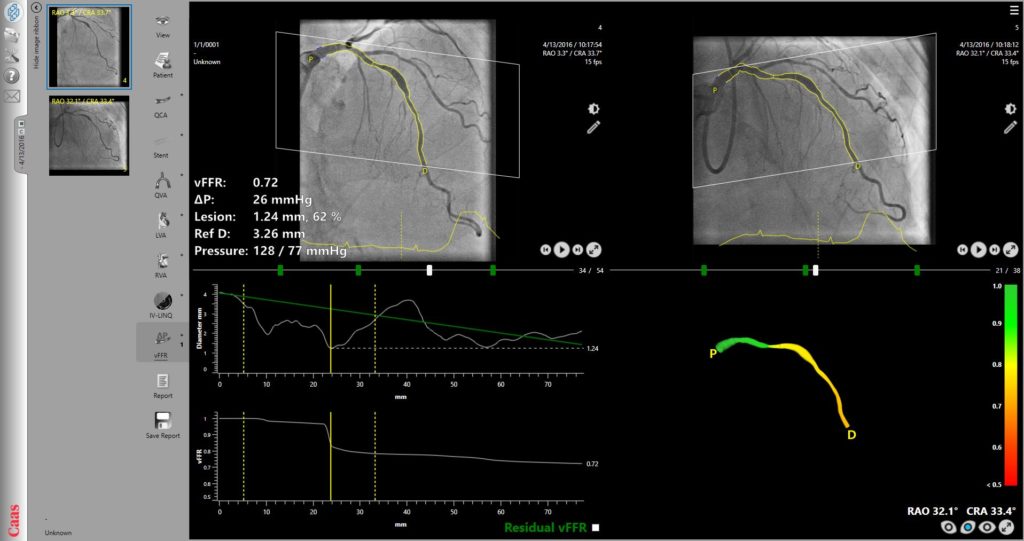
vFFR—FFR derived from routine coronary angiography—eliminates these issues and could be a cost saving and easier approach, Daemen said. In his words, vFFR is a 3D, angiography-based FFR index, calculated based on the 3D reconstruction of the coronary anatomy.
The technology requires two orthogonal angiographic projections that allow a 3D reconstruction of the vessel, combined with an input boundary condition for the pressure, which in this case is the aortic root pressure. Taken together these provide an instantaneous calculation of the vFFR over the specific segment of interest based on simplified computational fluid dynamics embedded in the algorithm.
Studies have already validated the use of the vFFR algorithm in practice. Among them is FAST I, the initial validation study of the technique. This study was led by Daemen and Erasmus colleague Ken Masdjedi, and validated the software on 100 patients undergoing invasive pressure wire based FFR recordings. This showed that vFFR, as calculated using CAAS vFFR, had a high linear correlation to invasively measured FFR. “We were able to demonstrate a high diagnostic performance of the technology with an excellent correlation as compared to pressure wire based FFR in this series of 100 patients,” says Daemen. The study also showed that the diagnostic accuracy of vFFR in identifying lesions with an FFR ≤0.80, which was higher when compared with 3D-quantitative coronary angiography (QCA).
Further to FAST I was the FAST EXTEND retrospective study, in which investigators assessed 296 patients using a similar approach to validate the use of vFFR in an extended cohort of patients. “We were able to confirm the diagnostic accuracy and correlation figures found in FAST I, and were also able to demonstrate a very low inference of variability,” comments Daemen, of the results.
These studies led to FAST II, which sought to validate the 3D QCA-based CAAS vFFR software in a prospective multicentre setting as compared to invasively measured pressure wire-based FFR. The study included patients aged ≥18 years, presenting with stable angina or non-ST segment elevation myocardial infarction (NSTEMI), in whom pre-PCI FFR was performed in at least one coronary artery with intermediate stenosis.
 Daemen explained that key features of the FAST II trial included that the study tested a larger set of angiograms, as recorded at different institutions around the globe with the vFFRs calculated by both a blinded corelab as well as local operators. Patients with ST-elevation myocardial infarction (STEMI), cardiogenic shock or severe haemodynamic instability, adenosine intolerance or previous coronary artery bypass grafting (CABG) were excluded, as were those with ostial or left main lesions with an estimated diameter of stenosis >50%, a thrombus containing lesion, or excessive overlap or tortuosity precluding vFFR computation.
Daemen explained that key features of the FAST II trial included that the study tested a larger set of angiograms, as recorded at different institutions around the globe with the vFFRs calculated by both a blinded corelab as well as local operators. Patients with ST-elevation myocardial infarction (STEMI), cardiogenic shock or severe haemodynamic instability, adenosine intolerance or previous coronary artery bypass grafting (CABG) were excluded, as were those with ostial or left main lesions with an estimated diameter of stenosis >50%, a thrombus containing lesion, or excessive overlap or tortuosity precluding vFFR computation.
Running from October 2018 to September 2020, the six-centre study identified 391 eligible patients. Of these, 337 were eligible for corelab vFFR, and 334 were available for final analysis. The final patient group where mainly male (73), with an average age of 66 years (±12 years). Predominantly, patients presented with stable angina (83%), and a smaller proportion with NSTEMI (12%) and unstable angina (5%).
Results of FAST II appear to reinforce the FAST I and FAST EXTEND findings, Daemen commented, showing a high diagnostic performance of vFFR both when calculated on-site and by a blinded corelab. The data presented at EuroPCR show that vFFR achieved a mean value of 0.83 ± 0.09 when calculated by a blinded corelab and 0.82 ± 0.10 when calculated locally at the individual sites. Mean FFR, the reference standard was 0.83±0.08 . Analysis also showed a high degree of accuracy 90% vs. 83%, sensitivity 81% vs. 71%, and specificity 95% vs. 89%, in the analysis carried out in the corelab and on-site.
“The good thing is that we were able to confirm the findings of FAST I and FAST EXTEND, in the sense that we found a very good correlation between Core Lab vFFR and pressure wire based FFR, as well as for site-calculated vFFR and pressure wire FFR, with very promising sensitivity and specificity figures, very much in line with the initial study,” said Daemen, discussing the findings. “We were able to extend these findings to a population as encountered in different sites across the globe, where image acquisition might be different and where different image intensifiers were used.”
Considering the case that these results make for the more widespread use of vFFR in clinical practice, Daemen said that ease of use of the system could aid its uptake. “The nice thing here is that the workflow is very easy,” he said. “The system automatically detects the optimal frame in both projections, which is quite convenient. The system has very accurate contour detection, there was a need to correct the contours in less than 9% of the vessel segments in the study. That is something that is specifically of interest because this has never been published by any of the alternative angio-based indices.”
One important message Daemen highlighted is the need for good image quality—“People really need to make sure that they make good quality angiograms that allow the software to function properly,” he said.
Turning to future areas for study, Daemen said that it is important to consider how the technology would perform in routine practice when used online. “In FAST II we assessed vFFR offline, either in the site or in the corelab, but that does not necessarily reflect the performance in a routine practice,” he commented, adding that there is a need for outcome data comparing the use of the technology to a standard reference like FFR or instantaneous wave-free ratio (iFR). This will be studied in FAST III—an international multicentre randomised outcome trial that is being carried out across 32 sites in Europe with the aim to enrol over 2,200 patients.










