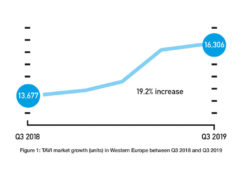
Thirty-day data presented at EuroPCR (22 May—25 May, Paris, France) show consistently positive patient outcomes for patients undergoing transcatheter aortic valve implantation (TAVI) with Sapien 3. The results—involving almost 2,000 severe, symptomatic aortic stenosis patients at intermediate-risk of open-heart surgery—demonstrated consistency with those results achieved in earlier controlled clinical trials in a limited number of hospitals.
The propensity-matched analysis comparing real-world data were collected from the Society of Thoracic Surgeons and American College of Cardiology (STS/ACC) Transcatheter Valve Therapy (TVT) Registry with outcomes of patients enrolled in the PARTNER II studies of the SAPIEN 3 valve. They were presented at EuroPCR by E Murat Tuzcu (The Heart & Vascular Institute, Cleveland Clinic Abu Dhabi, Abu Dhabi. Dubai).
Outcomes in a total of 1,956 intermediate-risk patients in the STS/ACC TVT Registry were compared with those of 652 intermediate-risk patients enrolled in the PARTNER II S3i study and 652 patients enrolled in the SAPIEN 3 intermediate-risk continued access program (S3iCAP) at 30 days. All patients were treated via the transfemoral access route.
At 30 days, the rate of all-cause mortality was 0.9% in S3i (N = 652), 0.9% in S3iCAP (N = 652), and 0.8% in TVT Registry IR (N = 1,956). The rate of all-cause stroke in these data sets were, respectively, 2%, 2.3%, and 2.2%.
Additionally, the post-approval data from the TVT Registry demonstrated a low rate of moderate to severe paravalvular leak, as well as a two-day average length of stay.
Tuzcu comments: “These data comparing real-world outcomes with clinical study results in intermediate-risk patients treated with the Edwards Sapien 3 transcatheter heart valve demonstrated comparably positive outcomes including high survival rates and low rates of stroke.”
Larry L Wood, Edwards’ corporate vice president, transcatheter heart valves, comments: “We are very pleased that, as the Sapien 3 valve therapy was introduced and used by a broader number of US clinicians in a real-world environment, the excellent patient outcomes reported from earlier clinical trials were preserved. Even as more hospitals offered transcatheter aortic valve replacement with the Sapien 3 valve, the procedure was generalizable and effective. These registry data are an important indication that, moving from a rigorously controlled environment of a clinical trial, to continued access and then to a commercial environment, positive patient results can be maintained when hospitals offer broader access to patients in need.”