
New evidence offers fresh insights into the merits of dual antiplatelet therapy (DAPT) following coronary artery bypass graft (CABG) surgery and calls into question current guideline recommendations for anticoagulation post-bypass, according to investigators.
TOP-CABG and TACSI were among two hot line trials presented during the final day of the 2025 European Society of Cardiology (ESC) congress (29 August–1 September, Madrid, Spain), both focusing on anticoagulation strategies following bypass surgery.
The TACSI trial investigated whether 12 months of DAPT with ticagrelor and aspirin reduces the risk of cardiovascular outcomes compared with aspirin alone in patients with acute coronary syndrome (ACS) undergoing CABG. At 12 months, the incidence of major adverse cardiovascular events was similar with DAPT and aspirin alone, but major bleeding increased with DAPT, the trial’s investigators showed.
The TOP-CABG trial, meanwhile, compared 12 months of DAPT with 12 months of de-escalated DAPT—DAPT for three months followed by aspirin monotherapy for nine months—in patients after CABG, assessing the impact of the two strategies on rates of graft occlusion alongside the risk of clinically relevant bleeding. The trial showed that the rate of graft occlusion was non-inferior between de-escalated DAPT and standard DAPT, but clinically relevant bleeding was less frequent with de-escalation.
“Current guidelines recommend dual antiplatelet therapy after CABG in patients with acute coronary syndrome, but these guidelines are based on very thin data, mainly sub-studies of larger ACS studies and investigations in PCI [percutaneous coronary intervention] patients,” Anders Jeppsson (Sahlgrenska University Hospital, Gothenburg, Sweden), the principal investigator in the TACSI trial told journalists at a press conference at the ESC meeting.
TACSI, an investigator-initiated pragmatic, open-label, registry-based randomised trial conducted in 22 cardiothoracic surgery centres in Sweden, Denmark, Norway, Finland and Iceland, randomised patients undergoing their first isolated CABG 1:1 to either DAPT (ticagrelor 90mg twice daily plus aspirin 75mg once daily) or aspirin only (75–160mg daily according to local protocols) for 12 months.
The primary efficacy endpoint of major adverse cardiovascular events (MACE) was a composite of all-cause death, myocardial infarction, stroke or new coronary revascularisation within 12 months, alongside a primary safety endpoint of major bleeding.
The 2,201 patients included had a mean age of 66 years and 14.4% were women. The primary endpoint of MACE occurred in a similar proportion of patients in each group: 4.8% of patients in the DAPT group and 4.6% in the aspirin only group, whilst major bleeding was more frequent in the DAPT group (4.9% vs. 2%).
A key secondary endpoint of net adverse clinical events (the primary endpoint plus major bleeding) was higher in the DAPT group than in the aspirin group (9.1% vs. 6.4%). A total of 0.7% of patients with DAPT and 0.2% with aspirin only died during the first year after randomisation.
“Dual antiplatelet therapy with ticagrelor and aspirin was not more effective than aspirin alone to prevent cardiovascular events but increases the risk for major bleeding,” said Jeppsson of the trial’s findings, which are published simultaneously in the New England Journal of Medicine. “The results question current guideline recommendations, which recommend 12 months of dual antiplatelet therapy in patients undergoing CABG after acute coronary syndrome,” he added.
With a focus on graft occlusion, TOP-CABG, a double-blind, parallel-controlled randomised trial conducted at 13 hospitals in China, sought to compare how the effects of de-escalated DAPT and a standard 12-month DAPT regimen influenced patency and bleeding events for one year after CABG procedures.
Patients older than 18 and younger than 80 years old were recruited if they were undergoing planned CABG for the first time with at least one saphenous vein graft and randomised 1:1 to de-escalated DAPT (ticagrelor 90mg twice daily plus aspirin 100mg once daily for three months, then placebo twice daily plus aspirin 100mg once daily for nine months) or to DAPT (ticagrelor 90mg twice daily plus aspirin 100mg once daily for one year).
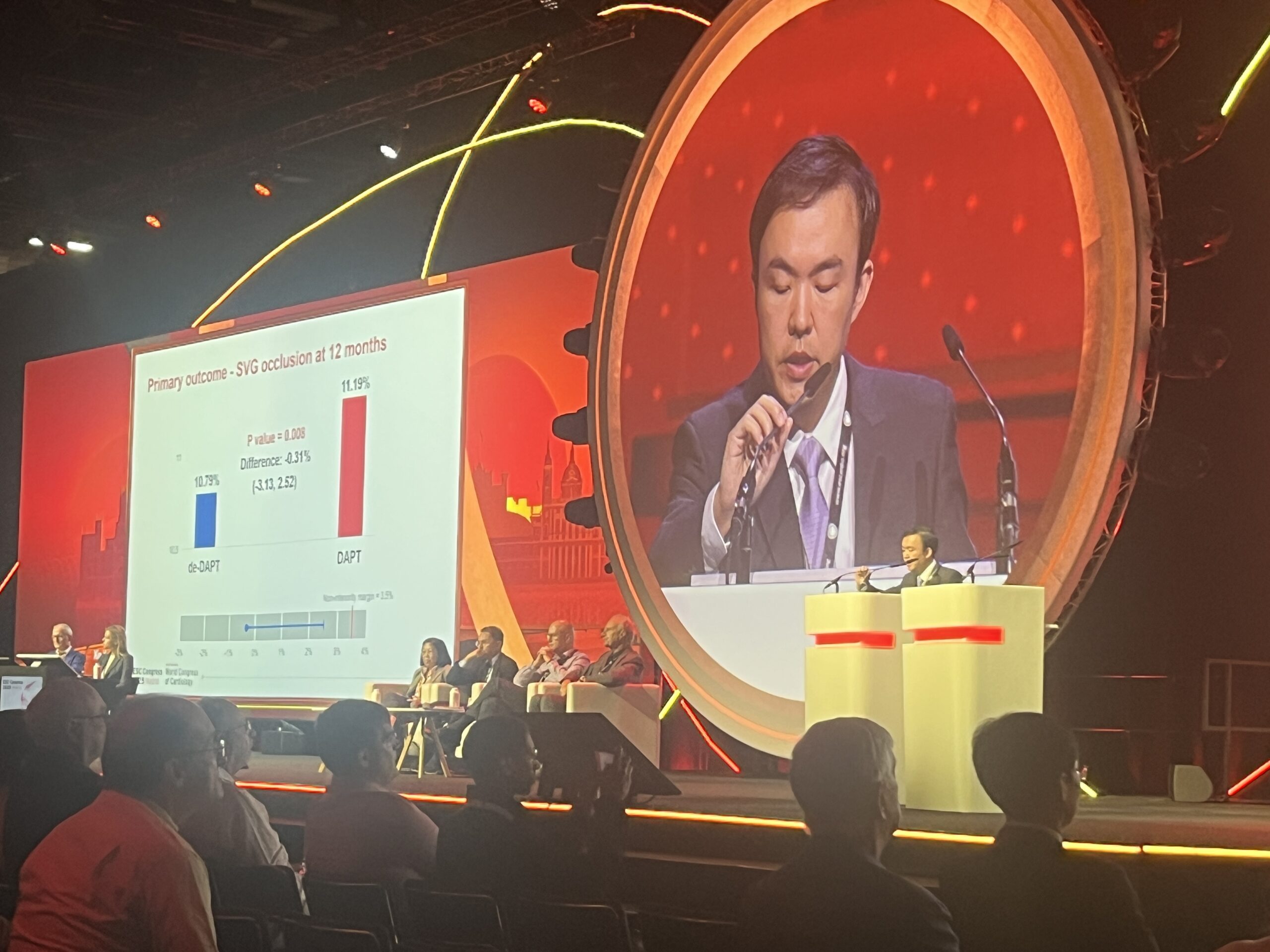
The primary non-inferiority efficacy endpoint was 100% occlusion of the saphenous vein graft within one year after CABG at the per-graft level, with occlusion assessed by coronary computed tomography angiography (CCTA) or coronary angiography, with a prespecified non-inferiority margin of 3.5%. The primary superiority safety endpoint was clinically relevant bleeding at the per-patient level (Bleeding Academic Research Consortium [BARC] classification ≥2) within one year. Among the 2,290 patients enrolled in the trial, 20.6% were female and the mean age was 61.5 years.
Sharing the results at ESC 2025, Xin Yuan (Fuwai Hospital, Beijing, China) reported that non-inferiority was demonstrated for the primary efficacy endpoint of graft occlusion, which occurred in 10.79% of patients’ grafts in the de-escalated DAPT group and 11.19% in the DAPT group, whilst the primary safety endpoint of clinically relevant bleeding was less frequent with de-escalated DAPT vs. DAPT (8.26% vs. 13.19%). There was no difference between the groups for secondary outcomes including graft failure, any graft stenosis, any graft occlusion or major adverse cardiac and cerebrovascular events, he reported.
“In the largest CABG trial to date, a de-escalation strategy offered a better balance between graft patency protection and bleeding risk than DAPT,” said Yuan. “These findings may help to inform future guidelines regarding the benefits of a shorter period of DAPT during the early phase after CABG.”









