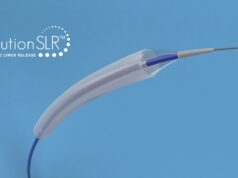
Cardionovum‘s Restore paclitaxel-coated balloon for coronary applications has received market approval for use in China. It can be used in treating two indications: in-stent restenosis and small vessel disease. A joint press release from Cardionovum and China Grand Pharmaceutical and Healthcare Holdings states that the Restore device features the company’s SafePax next-generation, stable, homogeneous, paclitaxel coating. It says that SafePax is an amorphous, noncrystalline matrix that is not affected by mechanical stress due to elastic, lipophilic, polymeric excipients.
The joint statement also says that the approval was supported by results from two multicentre, randomised controlled trials in Chinese populations: RESTORE ISR CHINA and RESTORE SVD CHINA.
RESTORE ISR CHINA consisted of 240 patients with coronary ISR at 12 sites in China. The performance of the Restore DCB matched the Sequent Please DCB (B. Braun Interventional Systems) on both in-segment and in-device late lumen losses at nine months after the procedure and on rates of target lesion failure at one year.
RESTORE SVD CHINA studied 262 patients with coronary small vessel lesions at 12 sites. It found that the Restore DCB was non-inferior to the latest-generation Resolute zotarolimus-eluting stent (Medtronic) on percent-diameter restenosis at nine months, as well as on rates of target lesion failure at one and two years.
Additionally, according to the press release, a substudy of patients with very small vessels (≥ 2mm and < 2.25mm) demonstrated that Restore was successfully employed with a very low procedural complication rate and no death, myocardial infarction, or thrombotic events.
Marina Izzo, CEO of Cardionovum, says in the statement: “At Cardionovum, we are passionate about driving the future and Restore is one of our most innovative products. I am very proud of the Chinese elite investigators whose hard work laid the foundation for this approval. RESTORE is the first and only DCB available in China to treat SVD. This is a great honour for us and a validation of our focus on performance-driven quality.”
Cardionovum is a medical technology company based in Bonn, Germany, that develops and commercialises medical devices such as new generation of DCB and drug-eluting stents for the treatment of coronary and peripheral artery disease. China Grand Pharmaceutical and Healthcare Holdings is an investment holding company engaged in the development, manufacture and sale of pharmaceutical preparations, medical devices, pharmaceutical intermediates, specialised raw materials and healthcare products.