The independent Data Safety Monitoring Board (DSMB) has found no significant safety concerns with BioCardia’s CardiAMP trial results. It recommends that the study continue as planned.
A pre-specified interim analysis considered safety outcomes for the first 10 patients treated in the phase III trial of its investigational CardiAMP cell therapy product.
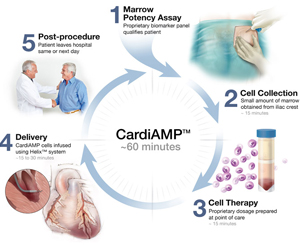
This ongoing multicentre, double-blinded, randomised (3:2) sham-controlled phase 3 trial is expected to enrol 260 patients at up to 40 centres nationwide. The trial is evaluating CardiAMP cell therapy in adult patients with heart failure that develops following a heart attack.
The primary efficacy endpoint is a significant improvement in six-minute-walk distance at 12 months post-treatment. It also incorporates the impact of major adverse cardiac events and other clinically meaningful events.
Study subjects must be diagnosed with New York Heart Association (NYHA) Class II or III heart failure as a result of a previous heart attack.
The national co-principal investigators of the CardiAMP trial are Raval and Carl Pepine of the University of Florida, Gainesville, USA.
An “important milestone” for CardiAMP
Amish Raval, associate professor of cardiovascular medicine for the University of Wisconsin School of Medicine and Public Health, Madison, USA, and co-national principal investigator for the ongoing CardiAMP Heart Failure trial, states, “The CardiAMP cell therapy program has hit another very important milestone. This minimally invasive, point of care approach has the potential to revolutionise the treatment of ischaemic heart failure.
“We are now recruiting for the randomised, controlled pivotal trial, which is designed to test the hypothesis that transendocardial delivery of autologous bone marrow mononuclear cells to patients, who are pre-selected with high bone marrow potency markers, will improve six-minute-walk distance and other clinically relevant endpoints.”
The company anticipates filing an investigational device exemption (IDE) supplement to add an interim efficacy readout. This will take place in the fourth quarter of 2018. BioCardia expects top line data in the fourth quarter of 2019.













