Tag: biocardia
BioCardia submits 510(k) approval for Morph DNA steerable introducer sheath family
BioCardia has submitted a 510(k) for approval of its patented Morph DNA steerable introducer sheath ...
CardiAMP Heart Failure Trial continues with first patient randomised in Covid-19 era
BioCardia has announced that it has resumed cases in the CardiAMP Heart Failure Trial. The first...
Positive preclinical results supporting investigational new drug application for anti-inflammatory cell therapy for heart failure
BioCardia has announced data from a recent animal study performed that, according to a press rel...
BioCardia gets FDA clearance for catheter to guide Helix delivery system
BioCardia has received US Food and Drug Administration (FDA) 510(k) clearance for its Morph DNA ...
Early treatment with Helix system improves ejection fraction and symptoms
The Helix biotherapeutic delivery system (Biocardia), which delivers cell therapy to patients wi...
BioCardia submits 510(k) application to US FDA for its Avance steerable introducer for transseptal access to heart
BioCardia has announced its 510(k) submission for US FDA clearance of the Avance steerable intro...
Mark Schwartz become vice president of Clinical Affairs at BioCardia
BioCardia has announced that Mark Schwartz—a 25-year veteran of cardiovascular device development—ha...
BioCardia and CellProThera to partner for clinical trial and marketing in Singapore of early stem cell therapy
CellProThera and BioCardia announced an agreement to expand their current collaboration to the S...
BioCardia phase III CardiAMP trial passes interim safety analysis
The independent Data Safety Monitoring Board (DSMB) has found no significant safety concerns wit...
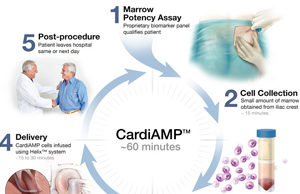
CardiAMP Heart Failure trial to further evaluate use of regenerative therapy in heart failure patients
BioCardia has revealed the trial design for its pivotal Phase III CardiAMP Heart Failure Trial at th...
Transendocardial delivery results in greater myocardial stem cell therapy retention
According to a study published in the International Heart Journal, delivery of stem cell therapy...
First patient treated with CardiAmp cell therapy for ischaemic heart failure in Phase III trial
Johns Hopkins Medicine, Baltimore, USA, the Maryland Stem Cell Research Fund (MSCRF) and BioCardia h...