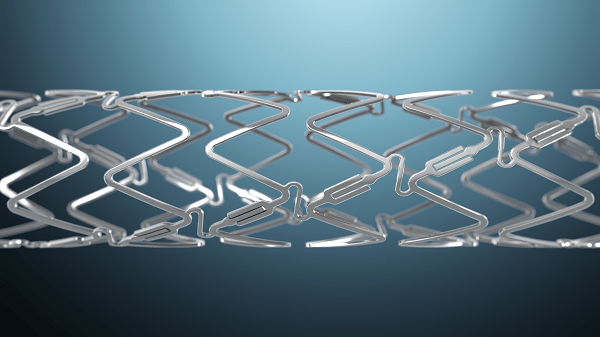
Elixir Medical has announced completion of enrolment in the BIOADAPTOR randomised controlled trial (RCT), evaluating the Dynamx coronary bioadaptor system, a drug-eluting coronary artery implant that adapts to vessel physiology, allowing for the restoration of normal vessel functions.
The BIOADAPTOR RCT is a multicentre, randomised, single-blind study enrolling 444 patients from 35 centres in Japan, Europe and New Zealand treated with the Dynamx Bioadaptor in a 1:1 randomisation to the Resolute Onyx drug-eluting stent (Medtronic). The primary endpoint of the study is target lesion failure (TLF) at one year. Secondary endpoints include measures of the implant’s ability to accommodate vessel growth from disease progression and restore vessel pulsatility in an imaging subset, as well as the incidence of major cardiovascular events.
“The bioadaptor is a revolutionary innovation in percutaneous coronary intervention and we are enthusiastic about studying the device’s ability to accommodate and restore vessel movement and function,” said Shigeru Saito, director of the Cardiology and Catheterization Laboratory at Shonan Kamakuru General Hospital, Kanagawa, Japan, and principal investigator of the BIOADAPTOR RCT. “Despite the challenges presented by the COVID-19 pandemic, we are proud that we were able to enrol the trial in about one year.”
According to Elixir Medical, the device incorporates “uncaging elements” in its metallic implant design, allowing for the restoration of natural artery function and movement essential to cardiovascular performance. The Dynamx Bioadaptor is designed to improve clinical outcomes by adapting to vessel physiology and restoring positive adaptive remodelling, pulsatility and rotation, the company adds.













