Amplitude Vascular Systems (AVS)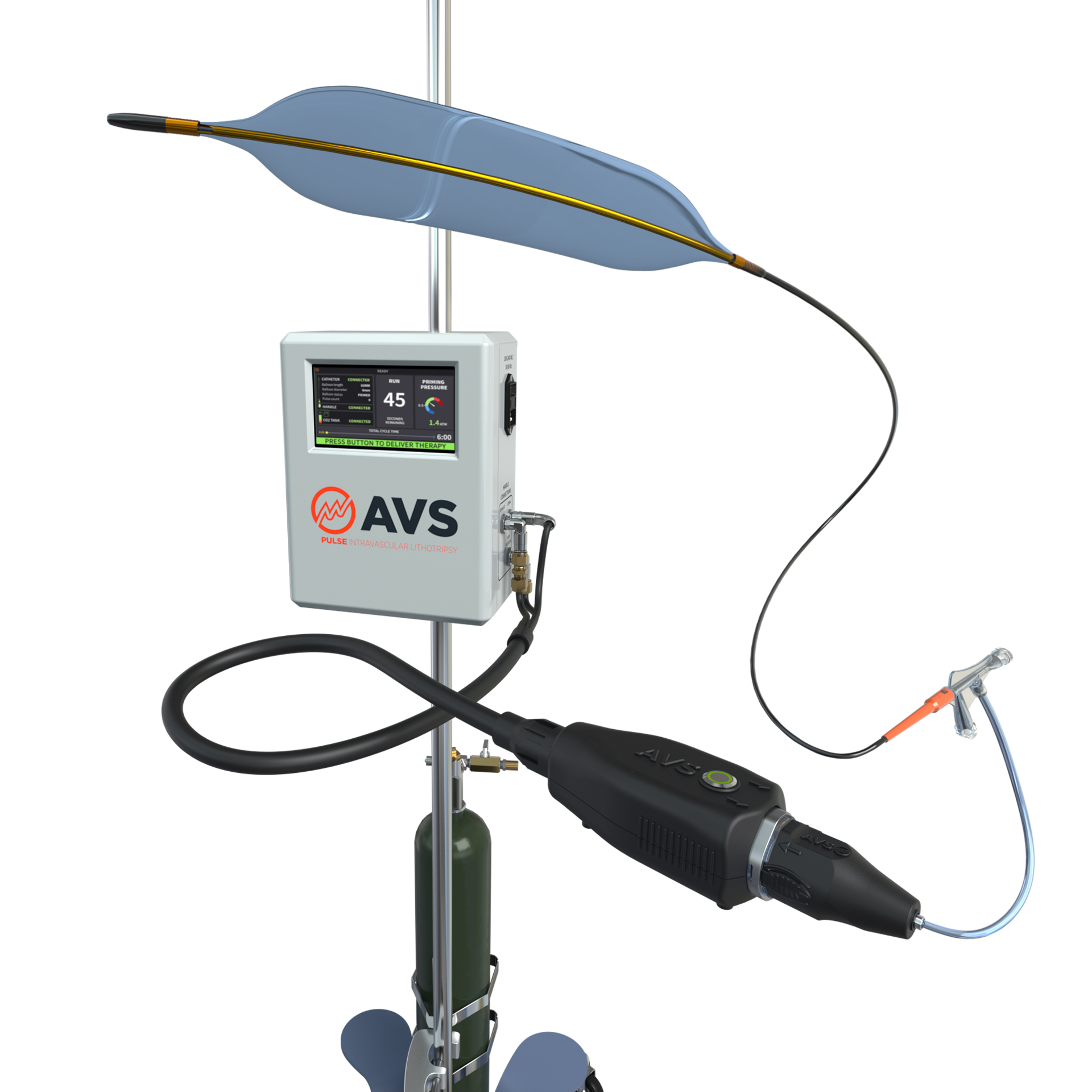 recently announced that it has completed a Series B round of financing of US$36 million. The funding will support the US peripheral commercial launch as well as US coronary and carotid investigational device exemption (IDE) trials for the company’s Pulse intravascular lithotripsy (PIVL) device.
recently announced that it has completed a Series B round of financing of US$36 million. The funding will support the US peripheral commercial launch as well as US coronary and carotid investigational device exemption (IDE) trials for the company’s Pulse intravascular lithotripsy (PIVL) device.
“We are pleased to see strong, sustained investor excitement around this technology,” said Mark Toland, chairman of the board for AVS. “Intravascular lithotripsy now represents a large and well-proven therapy that demands new solutions for patients with severely calcified arterial disease. This investor confidence reflects accelerated execution of critical company milestones in 2024, including the initiation of our US peripheral IDE. The commitment from our partners at BioStar Capital and Cue Growth Partners reinforces the great progress toward commercial approval with this technology in the IVL space.”
AVS began enrolling patients in October 2024 in the POWER PAD II US IDE trial, which is a prospective, single-arm, multicentre study evaluating the technical and clinical success of the Pulse IVL system for treating patients with calcific femoropopliteal arteries. The study will enrol up to 120 patients at 20 US sites and enrolment is expected to be completed mid-year 2025.
“AVS is the second company to conduct a peripheral intravascular lithotripsy pivotal IDE trial in the USA. By introducing a new, innovative treatment for calcified arterial disease, we can make a dramatic impact on patient lives and improve outcomes,” said interventional cardiologist Chris Metzger (OhioHealth Riverside Methodist Hospital, Columbus, USA), national principal investigator of the POWER PAD II study.
The funding will also support the company’s coronary US IDE study. “Because the Pulse IVL device uses a unique mechanism of action that eliminates the need for electrical emitters, the deliverability, crossability and efficiency are optimised for very challenging and tortuous coronary cases,” said Steven Yakubov, medical director of the OhioHealth Research Foundation (Columbus, USA) and member of AVS’ physician steering committee.
Lastly, the capital raised will support the US carotid IDE trial, as part of its partnership with the Jacobs Institute in Buffalo, USA, led by Adnan Siddiqui. “We are looking forward to studying the Pulse IVL system in carotids to improve stroke care. We believe the differentiated efficiency of this device versus other IVL catheters will be an important solution for our carotid disease patients, where a shorter treatment time is of utmost importance,” said Siddiqui.










