This advertorial is sponsored by Medtronic

The first case using the Prevail (Medtronic) paclitaxel-coated balloon catheter has been heralded by Bruno Scheller (University of Saarland, Homburg/Saar, Germany), a pioneer of drug-coated balloon (DCB) technology, as an important milestone for the use of balloon catheters for coronary angioplasty.
Scheller performed the first procedure using Prevail at Saarland University Hospital on 20 July, alongside Felix Mahfoud (University of Saarland, Homburg/Saar, Germany). As one of the forefathers of the DCB, Scheller commented that he is pleased to see continued research and investment in the development of DCB technology, as evidenced by Prevail.
“For me it is very nice to see that this technology is not static, that there are companies investing in and improving the technology,” he tells Cardiovascular News. “My intention is to bring this technology to much broader clinical applications than it is today”.
Scheller’s involvement in the first Prevail implantation takes the DCB back to its roots, given his work in developing DCB technology in the late 1990s, when he worked alongside Ulrich Speck (Berlin, Germany), first researching the use of contrast media in interventional procedures. The two were the first to prove the concept of the DCB, and led the first clinical trials to demonstrate the safety and efficacy of the technology.
“Leave nothing behind”
Scheller describes that the idea of “leaving nothing behind” by using a DCB is very attractive, especially in younger patients or those with long lesions. “If you have an acceptable result, without flow-limiting dissections, it is my experience that your vessel will look better after several months, and that is the beauty of this therapy,” he notes.
Medtronic’s Prevail, a paclitaxel DCB for the treatment of de novo small vessel disease or restenotic coronary artery lesions, is the latest entry into the coronary DCB sphere. The device features the proprietary drug-coating technology from Medtronic’s IN.PACT family of devices—FreePac™—which combines paclitaxel, a potent antirestenotic drug, with excipient urea to facilitate rapid drug transfer. “For the IN.PACT Admiral there has been a lot of data—it is the best investigated DCB in the peripheral arteries. This shows that this is one of the best coatings we have,” he says.
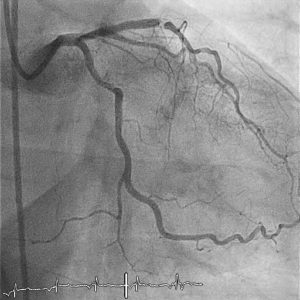 Offering details of the first Prevail case, Scheller explains that the patient is a 59-year-old male treated for coronary in-stent occlusion. The patient had a high cardiovascular risk profile, including the presence of arterial hypertension and hypercholesterolemia, a history of smoking, and obesity and had undergone percutaneous coronary intervention (PCI) four times in five years for three-vessel coronary artery disease, most recently receiving complex stent implantation for acute coronary syndrome.
Offering details of the first Prevail case, Scheller explains that the patient is a 59-year-old male treated for coronary in-stent occlusion. The patient had a high cardiovascular risk profile, including the presence of arterial hypertension and hypercholesterolemia, a history of smoking, and obesity and had undergone percutaneous coronary intervention (PCI) four times in five years for three-vessel coronary artery disease, most recently receiving complex stent implantation for acute coronary syndrome.

Detailing the procedure, Scheller explains that his and Mahfoud’s team achieved recanalisation via antegrade wire passage, followed by angioplasty with a 2.5mm balloon, and final lesion preparation with an NSE Alpha 3x13mm scoring balloon. Finally, local paclitaxel application was performed using a Prevail 3x20mm balloon, which Scheller says yielded a good primary angiographic result. “It looked very good. This is a state-of-the-art balloon system, and it worked as we expected,” he comments.

Deliverability has been a key focus in the development of the latest-generation device, which was preceded by Medtronic’s IN.PACT Falcon, and the device has been designed to offer greater pushability than predecessor DCBs.
Scheller described the deliverability of Prevail as being in line with his expectations, and agreed that improvements of rhe Prevail delivery system featuring Resolute Onyx DES PowerTrac™ Technology, bring Prevail on a par to the most trackable available DCBs in terms of its deliverability.
Scheller has welcomed the continued development of the DCB and Medtronic’s introduction of Prevail, which he sees as offering benefits from both the clinical and the device perspective. “It is nice to see that companies like Medtronic, who have invested a lot in stents and now see that there is an unmet clinical need in the coronary field and it makes sense to invest in such a programmes.”
Click here for more information regarding the Prevail DCB.













