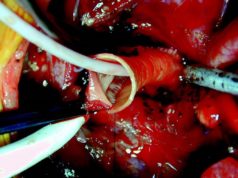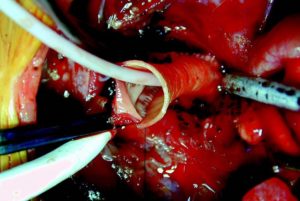
Admedus has announced positive data from an independent study into the performance of its tissue engineered bovine pericardial implant CardioCel, which is used in the repair of congenital heart defects in children.
Published in The Annals of Thoracic Surgery, the retrospective multicenter study of 501 implants in 377 patients showed that the CardioCel bioscaffold showed no calcification after five years, and that 96% of patients would not require reintervention.
Investigators also found that CardioCel has good durability when used for the repair of congenital heart defects, and performs comparably in the systemic and pulmonary circulations in neonates, infants, and older children.
A press release from the company also pointed out that CardioCell increased quality of life and offered cost savings to alternative implants.
The primary endpoint of the trial was CardioCel related surgical or catheter intervention. Secondary endpoints included implant related thromboembolism, residual shunt, infection, calcification leading to loss of function, and haemodynamic compromise. Mortality or reintervention was considered early if the event occurred within 30 days of implantation.
Cardiocel is a bioscaffold that uses Admedus’ Adapt technology, and is described by the company as a specially treated bovine tissue with zero DNA.