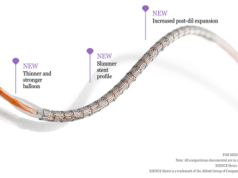
Two-year data from the ABSORB III study indicate that a bioresorbable vascular scaffold (Absorb, Abbott Vascular) is associated with a significantly higher rate of target lesion failure than is a permanent polymer everolimus-eluting stent (Xience, Abbott Vascular). However, a subanalysis of the study indicates that there are no significant differences in the rate of target lesion failure between Absorb and Xience if the scaffold is implanted in vessels ≥2.25mm.
Presenting the data at the 2017 scientific sessions of the American College of Cardiology (ACC; 17–19 March, Washington, DC, USA), Stephen G Ellis (Department of Cardiovascular Medicine, Heart and Vascular Institute, Cleveland Clinic, Cleveland, USA) reported that the superiority of the bioresorbable scaffold “is not likely to emerge before the bioresorption process is complete”, which is approximately three years. He added: “In consultation with the study’s principal investigators and the FDA, the landmark target lesion failure endpoint of ABSORB II/IV was revised from between one and five years in the initial protocol to between three and seven (or up to 10 years). This change allows unblinding of the clinical endpoints between one and three years in the ABSORB III and ABSORB IV trials.”
At the two-year follow-up point, data for 1,296 patients in the Absorb arm and for 671 patients in the Xience arm were available (randomisation used a 2:1 ratio). These showed that the rate of target lesion failure was significantly higher in the Absorb arm: 11% vs. 7.9% for Xience (p=0.03). This higher rate was largely driven by a significant increase in the rate of target vessel myocardial infarction with Absorb (7.3% vs. 4.9%, respectively; p<0.04).
However, these findings with Absorb may relate to the device being used in small vessels. While the protocol of ABSORB III mandated that only vessels between 2.5mm and 3.75mm should be treated with Absorb, clinicians were allowed to assess the size of vessels only using coronary angiogram. This led to 19% of ABSORB III study population, according to quantitative coronary analysis (QCA), having small vessels. Therefore, Ellis and colleagues performed a subanalysis that excluded patients with vessels <2.25mm. In this analysis, there were no significant differences in two-year rate of target lesion failure between Absorb and Xience: 9.4% vs. 7% (p=0.11).
Furthermore, Ellis commented that the implantation technique for Absorb has “evolved in recent years”, adding that “a growing body of evidence from the ABSORB clinical trials and registries suggest that optimal techniques may improve clinical outcomes”. He explained that this optimal implantation technique has been described as “PSP”, which stands for “predilation, appropriate vessel sizing, and high-pressure postdilation”. Given that this technique was not specified in the ABSORB III protocol and was subsequently not used in all patients receiving Absorb, Ellis et al performed another subanalysis to evaluate the impact of the “PSP” technique.
Based on this analysis, the use of PSP resulted in reductions in target lesion failure and definite/probable stent thrombosis compared with not using the technique. Furthermore, the rates of target lesion failure and definite/probably stent thrombosis associated with Absorb implanted with PSP were similar to those of Xience. Ellis said: “These results show that this device is generally comparable with the drug-eluting metal stent when the device is placed in appropriately-sized vessels and placed using appropriate procedural techniques.”
He concluded: “Longer term data from the ABSORB III/IV programme will determine whether better patient selection and technique improves short-term outcomes, and whether Absorb improves late outcomes compared with Xience.”
The three-year results of the ABSORB II study, which were presented at the 2016 meeting of the Transcatheter Cardiovascular Therapeutics (TCT; 29 October–2 November Washington, DC, USA), found that vasomotor reactivity was not significantly better with Absorb compared with Xience while late lumen loss was significantly worse. However, the PSP technique was not mandated in the study protocol.
Following the presentation of the ABSORB III results, the FDA sent a letter to healthcare professionals in the USA to advise them of the increased rate of major adverse cardiac events (at two years) compared with Xience. The letter reminds clinicians to avoid using the device in small vessel and to use the PSP technique. It also says that the FDA is now working with Abbott Vascular to better understand the higher rate of target lesion failure at two years with Absorb.