Abbott Vascular has written a letter to European physicians to say that from 31 May, its bioresorbable vascular scaffold (Absorb) will only be available through clinical registries at “selected sites and institutions”. The letter states that the company is working with “European Regulatory Agencies and the medical expert community” to collect more real-world data for the device. It also notes that “the situation will be reviewed” next year.
According to the letter, limiting the use of Absorb to registries “will enable systematic data collection to address raised from recent congresses about the three-year clinical data and analysis from ABSORB II regarding the frequency of scaffold thrombosis and the duration of optimal dual antiplatelet therapy (DAPT) after implantation”. The ABSORB II three-year data, as reported by Cardiovascular News, indicated that late lumen loss, device thrombosis, and target-vessel myocardial infraction were all significantly higher with Absorb compared with Abbott’s everolimus-eluting permanent polymer stent Xience.
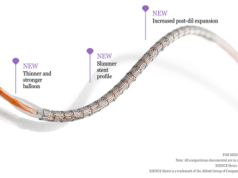
As well as addressing these concerns, the letter explains, the registry data will also “help to demonstrate the impact on clinical outcomes following changes to implantation technique”. Studies have indicated that a technique known as PSP—which stands for “predilation, appropriate vessel sizing, and high-pressure postdilation”—may help to improve outcomes with Absorb. Last month, at the 2017 scientific session of the American College of Cardiology (ACC; 17–19 March, Washington, DC, USA), Stephen G Ellis (Department of Cardiovascular Medicine, Heart and Vascular Institute, Cleveland Clinic, Cleveland, USA) reported that the two-year results of the ABSORB III/IV study indicate that the rate of target lesion failure is significantly higher with Absorb compared with Xience. However, he also said outcomes were similar between the devices when the correct technique was used.
Following Ellis’ presentation at the ACC, the FDA advised US physicians that the Absorb bioresorbable vascular scaffold (Abbott Vascular) may be associated with an increased rate of major cardiac events compared with Xience and to ensure that they used the correct technique for implanting the device.
To see the full letter from Abbott Vascular, see the story on CardioBrief.