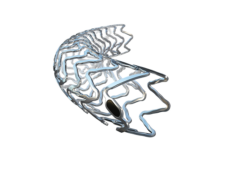
At a symposium at the 2016 Transcatheter Cardiovascular Therapeutics (TCT) meeting (29 October-2 November, Washington, DC, USA), physicians discussed their first experiences with a metallic bioresorbable scaffold (Magmaris, Biotronik). The BIOSOLVE-II trial had previously established the safety and clinical performance of Magmaris, which received the CE mark in June this year.
Additionally, during the symposium, physicians discussed interim results of Magnesium 1,000 programme. In order to help first implanters gain experience in the most effective possible use of Magmaris, the programme requires participating physicians to record each implantation. To date, over 500 implantations have taken place in 25 countries.
Physicians at the symposium also talked about why optimal patient profile and lesion selection is crucial when deciding whether to use a bioresorbable scaffold. Jacques Koolen (Catharina Hospital, Eindhoven, Netherlands) commented: “Cautious patient selection is key, and attention must be paid to lesion length, size and type to insure the best possible outcomes with Magmaris. Younger patients with de novo lesions in vessels with a diameter not smaller than 2.7mm are ideally suited for Magmaris. Evidence from trials and clinical practice demonstrate that with the right lesion, Magmaris leads to a simple and viable procedure which provides a very good outcome for the patient. This was established by the complete absence of scaffold thrombosis at 12 months observed in the BIOSOLVE-II trial. The upcoming BIOSOLVE-IV trial will shed more light on Magmaris’s efficacy in a wider patient population.”
BIOSOLVE-IV is a prospective, single-arm, multicentre registry looking at single de novo lesions with a primary endpoint of target lesion failure at 12 months. It has already enrolled the first of a projected 1,065 patients in Asia, Australia and Europe. Meanwhile, BIOSOLVE-III, a pivotal trial in 61 patients with single de novo lesions, has just completed its primary endpoint of procedural success during hospital stay.