Teleflex has announced that the Wattson temporary pacing guidewire limited market release has commenced at Columbia University Irving Medical Center (New York, USA). The first cases were performed by Tamim Nazif, Susheel Kodali and Isaac George.
Teleflex has announced that the Wattson temporary pacing guidewire limited market release has commenced at Columbia University Irving Medical Center (New York, USA). The first cases were performed by Tamim Nazif, Susheel Kodali and Isaac George.
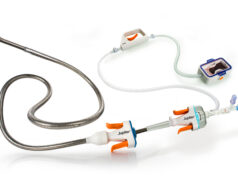
The Wattson temporary pacing guidewire supports both valve delivery and ventricular bipolar pacing during structural heart procedures, including transcatheter aortic valve implantation (TAVI) and balloon aortic valvuloplasty (BAV).
The device offers a procedural alternative designed to avoid potential complications, steps and costs associated with traditional right ventricular pacing. From its flexible distal pigtail shape to its multiple electrode, bipolar design, the Wattson temporary pacing guidewire is engineered to help reduce the risk of ventricular perforation while providing confidence in capture during rapid pacing.
“We are thrilled that our team at Columbia University Irving Medical Center was selected for the Wattson temporary pacing guidewire limited market release and are proud to have completed the first TAVI procedures with this important new device,” said Nazif. “The Wattson temporary pacing guidewire facilitated minimalist TAVI procedures with safe and reliable LV pacing throughout our first two days of cases, and we are eager to continue to use it moving forward.”
Teleflex received 510(k) clearance from the US Food and Drug Administration (FDA) for the Wattson temporary pacing guidewire in May of 2023.
“Providing interventionalists with a new tool specifically engineered to address unmet clinical needs frequently encountered during structural heart procedures is a significant milestone for Teleflex,” said Roger Graham, president and general manager of the Teleflex Interventional business unit. “The Wattson temporary pacing guidewire reflects our steadfast focus on filling gaps in existing technologies as well as our commitment to providing more options that further simplify minimalist TAVI and other structural procedures.”