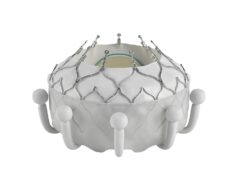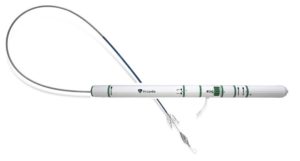
Venus Medtech has announced a collaboration to bring Pi-Cardia’s Leaflex technology to China.
Pi-Cardia’s Leaflex catheter performs mechanical scoring of valve calcification, restoring leaflets’ mobility and improving valve haemodynamics. The Leaflex catheter is to be used for patients who are not planning to undergo transcatheter aortic valve replacement (TAVR) and it can be a means to defer TAVR in patients who may be too young for the procedure. It can also be a preparatory step for improving the outcome of TAVR in heavily-calcified and bicuspid aortic valves.
In April, Pi-Cardia closed a US$27 million financing led by Sofinnova Partners, a leading European life sciences venture capital firm, following the successful completion of its first-in-human studies, which demonstrated acute safety and feasibility.
The strategic agreement with Venus Medtech will enable Pi-Cardia to pursue its global expansion by working on clinical trials, manufacturing and commercialising of its Leaflex technology, while concurrently continuing clinical trial activities in the USA and Europe. For Venus Medtech, this collaboration represents a bolstering of its portfolio of aortic valve products, enabling it to offer a unique non-implant based solution, the first of its kind in the world, to a rapidly growing Chinese market, the companies said in a press release.
“We are excited to collaborate with Pi-Cardia to bringing its unique Leaflex aortic valve repair technology to Chinese patients,” said Eric Zi, co-founder and chief executive officer of Venus Medtech. “The global aortic valve market is expected to reach US$9 billion over the next few years, and we see a very steep growth in these procedures in China. Our ability to offer a lower cost non-implant based solution for patients with calcified aortic valves is an important complement to our Venus TAVR platform, especially since patients in China are required to pay out of pocket for these procedures.”
“We are thrilled to work with a strong industry partner to make Leaflex a reality in China,” said Erez Golan, Pi-Cardia’s chief executive officer and founder. “With promising early clinical results, we are now ready to move to the next stage and establish the long term safety and efficacy of Leaflex as a standalone treatment for patients with aortic stenosis. We have an ambitious plan ahead of us, and we are dedicated to making Pi-Cardia’s technology the next revolution in the treatment of structural heart disease.”