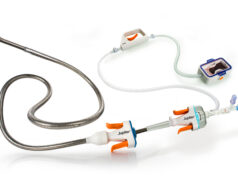
P&F USA, the US subsidiary of heart valve manufacturer P&F Products and Features, has announced that the US Food and Drug Administration (FDA) has approved initiation of the TRICAV II pivotal trial.
The study will evaluate the TricValve transcatheter bicaval valve system, a minimally invasive therapy for patients suffering from severe tricuspid regurgitation (TR) and right heart failure (RHF). TricValve has a breakthrough device designation, recognising its potential to offer a needed treatment option for patients with no surgical or commercially available transcatheter options.
TRICAV II pivotal trial is a randomised, controlled study comparing the TricValve system with optimal medical therapy (OMT) versus OMT alone. The FDA previously authorised TRICAV I, the early feasibility study evaluating the TricValve system in 110 patients at 50 US sites. TRICAV II advances its clinical development.
“The TRICAV II pivotal trial offers new hope for patients with severe TR who currently have no suitable surgical or transcatheter treatment options,” said Katharina Kiss, chief executive officer and co-founder of P&F, alongside Siegfried Einhellig, president and chief operating officer. “The TricValve system was developed specifically to address this underserved population with a safe, minimally invasive solution.”
“TricValve represents an important advancement,” said Samir Kapadia (Cleveland Clinic, Cleveland, USA), principal investigator along with Michael Reardon (Houston Methodist Hospital, Houston, USA). “Initiating this pivotal study is a significant milestone toward expanding treatment options in TR.”