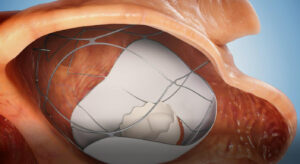
TruLeaf Medical has received Helsinki Ethics Committee approval in Uzbekistan to conduct a clinical trial using its RoseDoc transcatheter mitral valve replacement (TMVR) system in humans.
TMVR using the RoseDoc platform involves a two-stage procedure, which first sees a docking station implanted in the left atrium, followed by the implantation of an artificial biological mitral valve prosthesis several weeks later. The procedure is performed on a beating heart via two needle punctures without surgery or the use of a heart-lung machine.
As part of the preparations for human implantations in clinical trials in Uzbekistan, TruLeaf conducted additional implantations in large animals with the participation of Horst Sievert (CardioVascular Center, Frankfurt, Germany), who is expected to lead TruLeaf’s clinical trials.
TruLeaf Medical was founded in 2017 by Israeli entrepreneurs Benjamin Spencer, Nathaniel Benisho and Uri Rosenstein. Spencer and Benisho were involved in the development of the first ever transcatheter aortic bioprosthesis, initially within the Israeli company PVT, that was later acquired by Edwards Lifesciences.
Spencer, who is the CEO of TruLeaf Medical, said: “The main challenge with existing transcatheter TMVR technologies is achieving optimal anchoring of the valve prosthesis to the heart, given the complex anatomy and physiology of the native mitral valve. The RoseDoc TMVR platform is technically simple, safe, and has proven effective in long-term animal testing. Completely eliminating the leak prevents the progressive dilation of the heart, which otherwise worsens the leak in a vicious cycle, leading to further weakening of the heart muscle and intractable heart failure.
“Currently, patients with severe mitral valve leaks that are unresponsive to maximal medical treatment have no effective options. The vast majority of these patients are declined surgery due to prohibitive risk. The unique RoseDoc TMVR platform provides a potential lifeline for these patients.”
Oz Shapira, CEO of AllMeD Solutions, of which TruLeaf Medical is a subsidiary company, said: “As a heart surgeon who has performed hundreds of open-heart surgeries to treat leaky heart valves, the possibility of replacing the mitral valve through a simple and quick needle puncture operation is a true revolution that may offer a solution to millions who currently have no other option.”
“The first-in-human trial is both exciting and mission-critical for TruLeaf’s success. Given the outstanding results of the preclinical experiments, I am confident that TruLeaf’s innovative RoseDoc TMVR platform will perform exceptionally well in humans and eventually save countless lives of patients who currently have no alternatives. AllMeD Solutions will continue to demonstrate its ability to identify early-stage startups and leverage its vast knowledge, experience, and expertise in the med-tech space to lead these companies to engineering, clinical, and business success.”










