The heart teams at the Piedmont Heart Institute (Atlanta, USA) and the University of Virginia Health System (Charlottesville, USA) successfully performed the first two implantations in the USA of the Trisol transcatheter tricuspid valve replacement system (Trisol Medical) as part of a US Food and Drug Administration approved early feasibility study (EFS), led by principal investigator Isaac George (Columbia University, New York, USA).
The first case was performed in an 84-year-old woman with severe symptomatic tricuspid regurgitation (TR) at Piedmont Heart Institute through the right internal jugular vein. The TR level was reduced from severe to none and the patient was discharged from the hospital within two days after the procedure.
According to Pradeep Yadav, James Stewart and Vinod Thourani who conducted the first case, the procedure “marks a major milestone in the management of TR”.
“We were able to abolish the patient’s valvular heart disease via a minimally invasive procedure without the need for cardiopulmonary bypass,” the trio were quoted as saying in a press release issued by Trisol Medical. “The patient was mobilising within hours of the procedure and her recovery was steam-lined and expeditious.”
The second case was performed in a 77-year-old woman with severe symptomatic TR at the University of Virginia Health System. The TR level was reduced from severe to trace, and the patient was discharged from the hospital within two days after the procedure.
“The patient had a gratifying result with essentially elimination of her tricuspid regurgitation, and rapid recovery, along with significant and rapid improvement in her symptoms,” said Scott Lim, who conducted the second case. “We look forward to further investigation of the Trisol valve.”
“We are fortunate to collaborate with such skilled investigators. We are pleased with the preliminary outcomes and look forward to ongoing progress of the US EFS,” says Ron Davidson, Trisol’s CEO.
Shimon Eckhouse, Trisol’s chairman, further remarked: “There is a huge unmet need for a transcatheter solution to treat severe TR. Trisol’s promising initial clinical data instills confidence that Trisol can play a major role in this domain.”
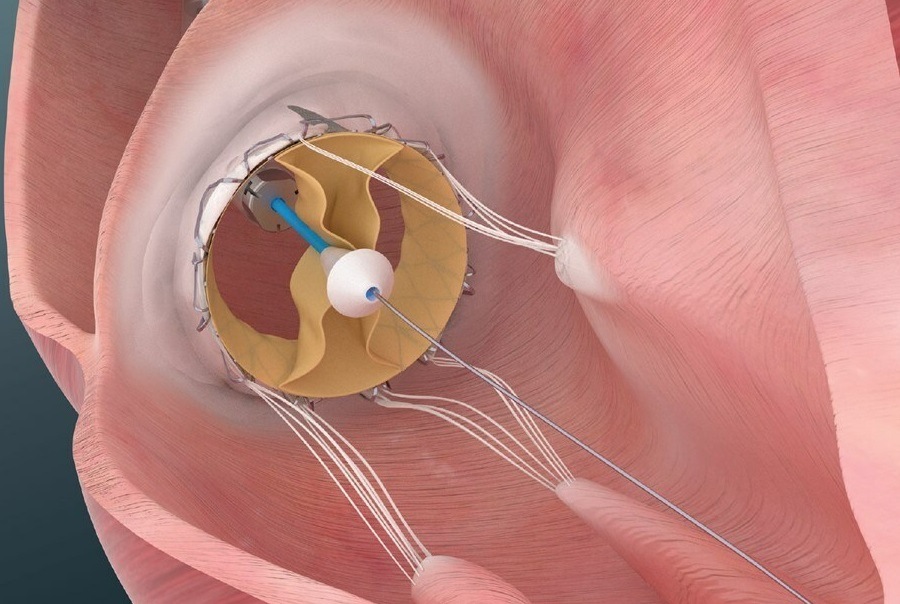
Trisol patented valve is comprised of a single leaflet, affixed by two commissures enabling it to function as a bileaflet valve. According to Trisol Medical, this design facilitates a slower closing of the leaflets, a feature that is intended to preserve the right ventricular function following the valve replacement. Trisol’s valve employs axial anchoring, that reduces the risk of conductive issues.
To date, Trisol Valve has been implanted in ten humans. Five of these implants were performed as part of the Israeli pilot study, led by Principal Investigator Ran Kornowski (Rabin Medical Center, Tel Aviv, Israel). Currently, the longest follow-up period exceeds two years. Trisol aims to complete its EFS and initiate pivotal studies in 2024.