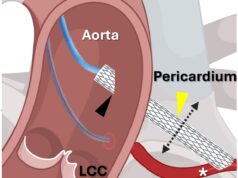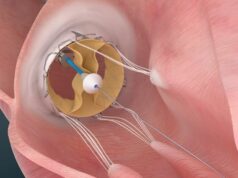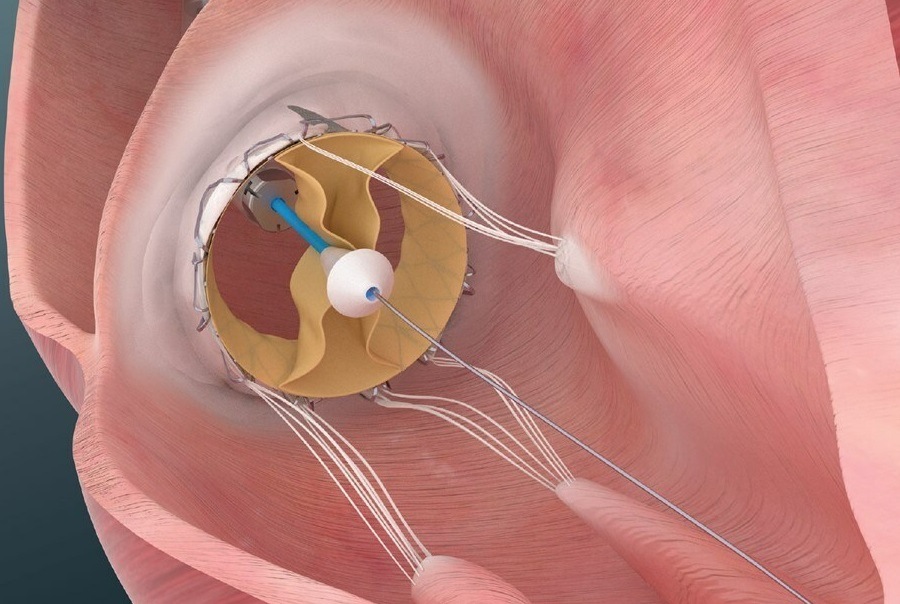
Trisol Medical has announced results from its US Food and Drug Administration (FDA)-approved early feasibility study evaluating the Trisol transcatheter tricuspid valve replacement (TTVR) system in patients with severe to torrential tricuspid regurgitation (TR).
To date, 22 patients with severe to torrential TR who were considered high risk for conventional surgery have been treated at US centres, including Piedmont Heart Institute, Vanderbilt University Medical Center, University of Virginia Health System, Columbia University Medical Center, and Cedars-Sinai Medical Center, using a trans-jugular (TJ) access approach. Enrolment in the TJ cohort is now complete, and the study is proceeding with continued enrolment using Trisol’s newly developed transfemoral access route.
Results from the study indicate a less than 5% need for permanent pacemaker at 30-day follow-up; considerable reduction in TR following implantation and notable improvements observed in quality of life (KCCQ), heart failure symptoms (NYHA class), 6-minute walk distance, right ventricular function, and cardiac output at 30-day and 12-month follow-up.
These results include patients with reduced right ventricular function, a large, high-risk subgroup associated with poorer outcomes and underserved by existing and emerging treatment approaches, Trisol says in a press release.
Pradeep Yadav (Piedmont Heart Institute, Atlanta, USA) commented: “Trisol brings several novel features from ease of use to recapturable anchors, broad size range, lower pacemaker rate and performance in dysfunctional right ventricles. We are very excited to investigate the next phase with its transfemoral delivery system and pivotal trial.”
“We are thrilled by these positive outcomes, which further validate the potential of our best-in-class technology to improve care for patients with severe TR. I would like to thank our clinical investigators and the entire clinical teams for their dedication and outstanding patient care,” said Ron Davidson, chief executive officer of Trisol Medical.
Shimon Eckhouse, chairman of Trisol, added: “Millions of Americans suffer from TR, and despite recent advances, effective treatment options remain limited for patients with severe TR. The Trisol valve was developed to address this significant unmet need and has the potential to redefine the standard of care.”