Latest data from the ALIGN AR trial, investigating the use of the Trilogy (JenaValve) transcatheter aortic valve implantation (TAVI) system in high-risk patients with symptomatic, severe aortic regurgitation, support the safety and efficacy of the device out to two years.
Torsten Vahl (NewYork-Presbyterian/ Columbia University Medical Center, New York, USA) presented two-year outcomes from the trial—a landmark prospective, single-arm investigational device exemption (IDE) study, intended to support US Food and Drug Administration (FDA) approval of the device—at TCT 2024 (27–30 October, Washington, DC, USA).
Design features of the Trilogy valve, including locators that clip onto native leaflets and enable secure anchoring in the absence of calcium, make the device well suited to the treatment of aortic regurgitation, investigators say, as well as helping to facilitate commissural alignment which can be important when treating aortic stenosis. Trilogy also features large-open cells that are designed to support future coronary access.
ALIGN AR, taking place at 20 sites throughout the USA, follows the clinical, echocardiographic, functional and quality of life outcomes annually out to five years among 180 patients with symptomatic, severe aortic regurgitation, deemed high-risk for surgery. One-year findings from the trial, reported at TCT in 2023, showed that Trilogy met its non-inferiority safety and efficacy endpoints.
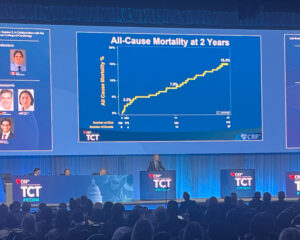
Vahl reported that at two years, all-cause mortality stood at 15.4%, continuing to fall within the threshold for non-inferiority. The rate of death attributed to cardiovascular causes stood at 6.2%. “This tells us that between year one and year two, the vast majority of patients, 11 out of 13, experienced non-cardiovascular death, that we attribute to the comorbidities of the patients,” said Vahl.
The haemodynamic performance of the valve also appears favourable according to Vahl, with mean effective orifice area (EOA) >2.5 cm2 and mean transvalvular gradient <5 mmHg, as well as no cases of haemodynamic valve deterioration.
Further study of the Trilogy valve is set to commence with the initiation of the ARTIST trial, which is due to begin enrolment shortly. The ARTIST trial is a randomised study comparing TAVI with Trilogy to surgical aortic valve replacement (SAVR) in non-high-risk patients with severe and moderate-to-severe aortic regurgitation.









