TRiCares has announce the second closing and completion of its Series C financing round, successfully raising a total of €51 million.
TRiCares has announce the second closing and completion of its Series C financing round, successfully raising a total of €51 million.
The proceeds of this financing will primarily be used to continue the development of the company’s transfemoral tricuspid heart valve replacement system—Topaz—up through to the application for a pivotal investigational device exemption (IDE) trial in the USA. To this end, TRiCares plans to initiate an early feasibility study across five centres in the USA and Canada in 2023.
The proceeds of this financing will also support the ongoing TRICURE first-in-human clinical study in Belgium as well as potential additional clinical trial applications elsewhere in Europe, along with further compassionate use implantations as appropriate.
The Series C financing was led by 415 Capital and joined by Bayern Kapital, the venture/growth capital company of the State of Bavaria in Germany, with strong support from existing investors Andera Partners, BioMed Partners, Credit Mutuel Innovation, GOCapital, Karista and Wellington Partners. Total gross proceeds were €51 million.
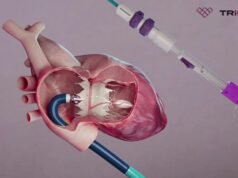
Topaz is an innovative device developed to help patients suffering from severe tricuspid regurgitation without the need for open heart surgery. The Topaz device is implanted through the patient’s femoral vein, and is designed specifically to fit the tricuspid valve anatomy and thus supports ease of positioning and functionality.
Helmut J Straubinger, chief executive officer of TRiCares, said: “Millions of patients with tricuspid regurgitation have no effective long-term treatment option, and their prognosis is very poor. The Topaz tricuspid valve replacement system is being developed with the aim of delivering a much-needed solution for these patients. With encouraging clinical signs, the strong support of world-class investors and our successful Series C financing, we look forward to the further development of the Topaz system with a focus on the necessary preparations for a pivotal IDE trial in the US along with the continued progress of our clinical activities in Europe.”
Georg Ried, managing director of Bayern Kapital, said: “Open heart tricuspid valve surgeries are among the riskiest curative procedures, with more than 99% of affected patients deemed unfit due to high mortality rates. With Topaz, TRiCares is developing an innovative product with excellent potential to fill this major gap in the treatment of valvular heart disease. We are very pleased to support TRiCares on its further course towards market approval.”
Frederik Groenewegen, general partner at 415 Capital, commented: “We believe that the technology developed by TRiCares has the potential to establish itself as the gold standard in the treatment of patients with tricuspid regurgitation and to restore the quality of life of millions of patients in the long term. We are impressed with the initial clinical cases with the Topaz system and look forward to helping the team bring this novel therapy to patients in the USA and Europe.”