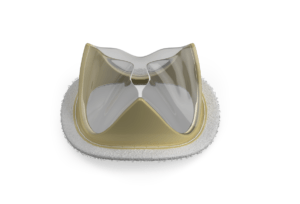
Foldax has announced the launch of the Tria mitral valve in India, marking the first time a polymer heart valve has been made commercially available to patients anywhere in the world.
Traditional valves made from animal tissue are prone to calcification and degradation, limiting their durability and often leading to repeat surgeries, especially for younger patients. Mechanical valves, while durable, are prone to thrombosis and require lifelong anticoagulation therapy that can impact a patient’s long-term quality of life. Foldax’s vision for its novel polymer heart valves is to address the limitations of tissue and mechanical valves by making its valves durable, with the future goal of avoiding the requirement for lifelong anticoagulation, the company says in a press release.
Mitral valve disease is a concern in India, with rising surgical volumes and increased need for long-term treatment solutions. The Tria mitral valve is positioned to offer a new option for patients seeking a durable solution without the lifestyle limitations of mechanical valves.
“We are honoured to work with India’s top cardiovascular surgeons and Dolphin Life Science to bring next generation medical solutions to the Indian population,” said Laxmikant Khanolkar, senior vice president of Foldax Asia and MEA. “This is the first in a series of heart valve solutions we intend to introduce into the market.”
As part of the launch, Foldax has initiated the Evolve training & certification programme to ensure high-quality outcomes and surgeon preparedness. Physicians who complete training will be certified to implant the valve at participating hospitals.
The Tria mitral valve in the USA is limited to investigational use only. The Tria mitral valve is approved for use in India by Central Drugs Standard Control Organisation (CDSCO).









