 Bivacor has announced that its total artificial heart (TAH) system has been accepted into the US Food and Drug Administration’s (FDA) total product life cycle (TPLC) advisory programme—known as the TAP programme.
Bivacor has announced that its total artificial heart (TAH) system has been accepted into the US Food and Drug Administration’s (FDA) total product life cycle (TPLC) advisory programme—known as the TAP programme.
The programme is designed to accelerate the development and patient access to high-impact medical technologies. Entry into the programme provides Bivacor with proactive, strategic engagement with the FDA throughout the entire product life cycle, from development to commercialisation, supporting more efficient, risk-informed decision-making, the company says in a press release.
“Acceptance into the TAP programme marks a major milestone not just for Bivacor, but for the field of mechanical circulatory support as a whole,” said Daniel Timms, founder and chief technology officer of Bivacor. “The Bivacor TAH has the potential to fundamentally redefine the standard of care for patients with end-stage heart failure. TAP access gives us a powerful framework for working hand-in-hand with the FDA to bring this technology to the patients who need it most.”
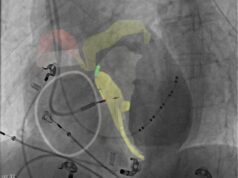
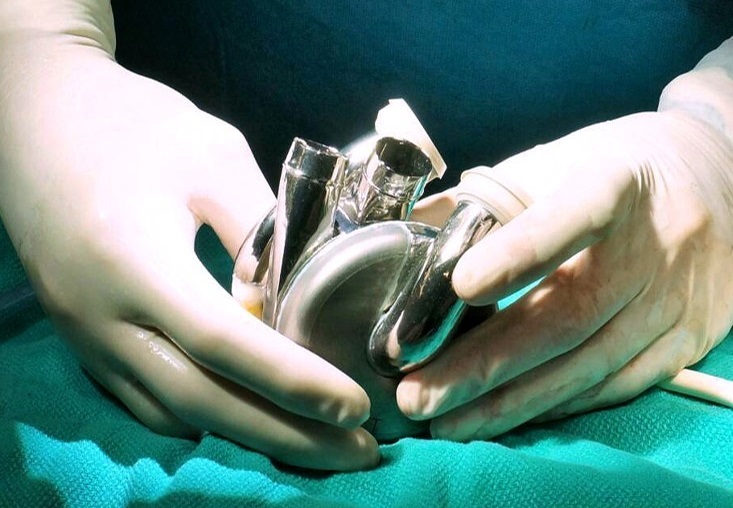
The Bivacor TAH is intended for use as a bridge to transplant in adults with severe, irreversible biventricular or univentricular heart failure, particularly for patients who cannot be treated with traditional left ventricular assist devices (LVADs). The system employs a magnetically levitated centrifugal pump, inspired by space and industrial technologies, which provides continuous, pulsatile, and physiologically responsive cardiac support.
The FDA’s acceptance letter to Bivacor emphasised that the TAP programme’s inclusion reflects the agency’s confidence in the technology’s potential to transform clinical practice. As part of the program, Bivacor will receive more regulatory guidance, earlier identification of scientific and evidentiary gaps, and greater coordination among stakeholders, including payers and patient advocacy groups.
“This isn’t just a vote of confidence in Bivacor, it’s a vote of confidence in the future of heart replacement technology,” said William Cohn, Bivacor chief medical officer and cardiac surgeon. “The TAP programme gives us the opportunity to collaborate with the FDA at a level that aligns with the urgency and magnitude of our mission to bring a durable, fully implantable artificial heart to patients with no other options.”
BiVACOR’s participation in TAP follows several recent milestones for the company, including its first-in-human implant as a bridge to support a patient awaiting a cardiac transplant. The company is now progressing toward expanded clinical trials in the USA and internationally.